Reports: ND555377-ND5: Molecular Understanding of Clay Swelling in Enhanced Oil/Gas Recovery
Yongsheng Leng, PhD, George Washington University
There are several molecular simulation studies on the structure and dynamics of CO2-H2O mixtures between charged clay surfaces,1-8 primarily due to the initial interest in the role of CO2 molecules intercalated into clay hydrates.
The project studies CO2-clay mineral interactions especially the effect of relative humidity (RH) on the swelling behavior of montmorillonite in wet CO2. Our approach is based on the equation of state (EOS) theory and Widom insertion method to determine the chemical potentials of CO2 and H2O in the bulk fluid mixture. The grand canonical Monte Carlo (GCMC) simulations are performed to study the intercalation of CO2 and H2O in different clay interlayers. For the equilibrium interlayer spacing determined by the GCMC simulations, molecular dynamics (MD) simulations are subsequently performed to study the adsorption structure and dynamics of different species (H2O, CO2 and Na+ ions) confined between charged clay surfaces.
We use a clay model of sodium-saturated Wyoming-type montmorillonite with the chemical formula Na0.75[Si7.75Al0.25] (Al3.5Mg0.5)O20(OH)4 for the clay surfaces. The CLAYFF1 force field for montmorillonite and SPC water model2 are used in simulations. For CO2 molecules, we use the elementary physical model (EPM2) with the flexible bond stretching and angle bending terms.3 Further, intramolecular bond stretch and angle bending terms are also considered in SPC water model4 to ensure full flexibility of these molecules and hydroxide components on clay surface.
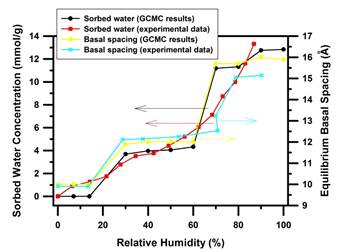
We consider typical CO2 geological sequestration conditions (T/P = 323 K/90 bar) in the molecular simulations. Under these T/P conditions experimental data are available for comparison.5-6 We find that initially water molecules are intercalated into clay interlayers. There is a threshold of RH for the clay swelling. Simulation results show that stable monolayer hydrates (1W) with a basal spacing around 12 Å are formed at RH = 30-60%. As RH is increased to 70% and above, bilayer CO2-H2O mixture with a basal spacing around 15-16 Å (2W) are more stable. The equilibrium basal spacing variation with RH is shown in Figure 1. These simulation results compare reasonably well with the in situ X-ray diffraction (XRD) experimental results by Loring et al.5 The sorbed H2O concentration exhibits stepwise increase in our simulations (Figure 1), in contrast to the continuous sorption curve measured in IR spectroscopy experiment.5 The discrepancy between simulation and the IR experimental results could be explained by the hydration-heterogeneity model. In this model, a distribution of individual interlayers with integral hydrations states (such as 0W, 1W, or 2W) exists at a given RH value. As the percent H2O saturation is increased starting from anhydrous state, the montmorillonite shifts from predominantly 0W, to a mixture of 0W + 1W, to predominantly ~1W, and so on, resulting in a continuous experimental sorption curve of H2O concentration, which actually "smears out" the stepwise curve predicted by our molecular simulations. From this perspective, we believe that the hydration-heterogeneity model may provide a reasonable interpretation to the continuous change of sorbed water concentration measured in experiment.
Figure 1. Variations of sorbed H2O concentration and equilibrium basal spacing as a function of the relative humidity (experimental data are from Loring et al.5)
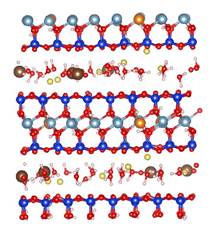
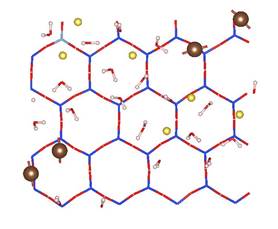
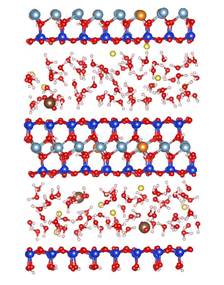
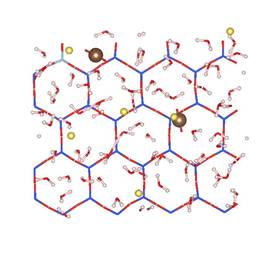
MD simulations show that CO2 molecules are partially hydrated in 1W hydration state, while sodium ions are fully hydrated due to its relatively high hydration energy with water molecules. Moreover, CO2 molecules hardly migrate into the first hydration shell of sodium ions in both 1W and 2W states. Figure 2 shows the typical snapshots of CO2 hydration configurations in two MD simulations. The first case corresponds to the equilibrium basal spacing of 11.9 Å at RH = 30% (1W). The second case corresponds to the equilibrium basal spacing of 15.9 Å at RH = 100% (2W). These snapshots clearly show that in 1W hydration state, the partially hydrated CO2 molecules can form dimer configurations (Figure 2a), while in 2W and above CO2 molecules tend to be fully hydrated (Figure 2b). CO2 dimers are frequently seen in the slipped parallel configuration rather than in the perpendicular T-shaped configuration. The diffusion coefficients of CO2 molecules are larger than those of water molecules and sodium ions. This comparably high mobility of CO2 molecules in clay interlayer, together with the low probability of CO2 participation in the first hydration shell of Na+ ions, implies that at least in a short term, direct CO2¨CNa+ interactions in clay interlayer are unlikely under typical CO2 geological sequestration conditions.
(a)
(b)
Figure 2. Snapshots of CO2¨CH2O mixture in Na-montmorillonite interlayer in two T/P/RH simulation cases. The left and right panels show the side and top views of the molecular configurations. (a) T/P/RH = 323 K/90 bar/30% with equilibrium basal spacing of 11.9 Å; (b) T/P/RH = 323 K/90 bar/100% with equilibrium basal spacing of 15.9 Å. Color scheme: oxygen ¨C red; hydrogen ¨C white; carbon ¨C brown; silicon ¨C blue; aluminum ¨C cyan; magnesium ¨C orange, and sodium ¨C yellow.
1. Cygan, R. T.; Liang, J. J.; Kalinichev, A. G., Molecular Models of Hydroxide, Oxyhydroxide, and Clay Phases and the Development of a General Force Field. Journal of Physical Chemistry B 2004, 108, 1255-1266.
2. Berendsen, H. J. C. P., J. P. M.;van Gunsteren, W. F.; Hermans, J., Interaction Models for Water in Relation to Protein Hydration. In Intermolecular Forces, Pullman, B., Ed. D. Reidel: Amsterdam, 1981; p 331.
3. Cygan, R. T.; Romanov, V. N.; Myshakin, E. M., Molecular Simulation of Carbon Dioxide Capture by Montmorillonite Using an Accurate and Flexible Force Field. Journal of Physical Chemistry C 2012, 116, 13079-13091.
4. Teleman, O.; Jonsson, B.; Engstrom, S., A Molecular-Dynamics Simulation of a Water Model with Intramolecular Degrees of Freedom. Molecular Physics 1987, 60, 193-203.
5. Loring, J. S.; Ilton, E. S.; Chen, J.; Thompson, C. J.; Martin, P. F.; Benezeth, P.; Rosso, K. M.; Felmy, A. R.; Schaef, H. T., In Situ Study of CO2 and H2o Partitioning between Na-Montmorillonite and Variably Wet Supercritical Carbon Dioxide. Langmuir 2014, 30, 6120-6128.
6. Loring, J. S., et al., In Situ Molecular Spectroscopic Evidence for CO2 Intercalation into Montmorillonite in Supercritical Carbon Dioxide. Langmuir 2012, 28, 7125-7128.