Reports: UR455293-UR4: Shedding Light on Diradical Forming Cyclizations and Cycloaromatizations
John D. Spence, California State University Sacramento
Goals: In the first year of this grant we have evaluated the effect of extended benzannelation orientation on C1-C6 and C1-C5 reaction channels of enediynes through combined kinetic and photochemical studies. To conduct these studies, terminal and phenylethynyl substituted quinoxalenediyne isomers were synthesized to examine how sterics combined with aromatic stabilization energy can influence reaction pathways of enediynes. The overall goal of this study is to examine structure-activity relationships associated with benzannelation in effort to selectively tune and optimize enediyne reaction channel.
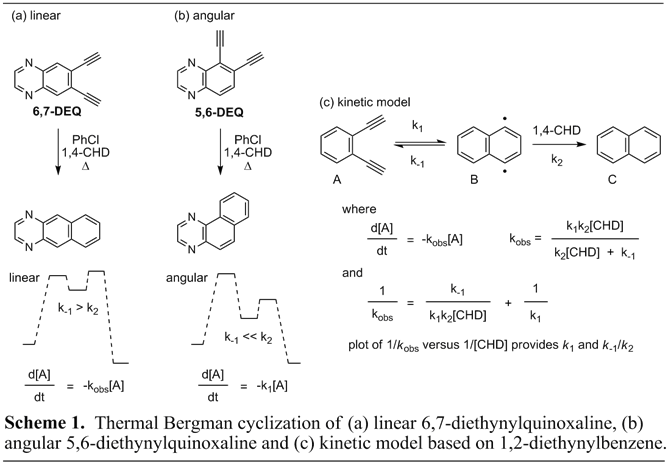
Results: To examine the effect of extended benzannelation orientation on Bergman cyclization energetics we conducted kinetic studies on isomeric quinoxalenediynes 5,6-diethynylquinoxaline (5,6-DEQ) and 6,7-diethynylquinoxaline (6,7-DEQ) (Scheme 1). Preliminary computational data suggested increased Bergman cyclization endothermicity upon linearly extended benzannelation in 6,7-DEQ as the gain of aromatic stabilization energy is attenuated. However, for angularly extended benzannelation present in 5,6-DEQ generation of a new aromatic sextet decreases endothermicity upon cyclization to the diradical. To gain experimental data to support our calculations kinetic studies were conducted to monitor rates of enediyne disappearance and product formation as a function of hydrogen atom donor concentration. Kinetic data was collected at 180 °C in chlorobenzene employing naphthalene as an internal standard with a 25 to 250 fold excess of 1,4-cyclohexadiene. The reaction course was monitored out to 2-3 half-lives by GC analysis, with our results presented in Table 1. From the data, the linear 6,7-DEQ shows a strong dependence on 1,4-cyclohexadiene concentration as predicted computationally. At low concentration of 1,4-cyclohexadiene (25-50 equivalents), however, the rate of product formation does not match rate of enediyne disappearance as polymerization of the diradical intermediate likely occurs. At higher concentrations of hydrogen atom donor (100 equivalents or greater) the rate of product formation matches the rate of enediyne disappearance (within experimental error) over the first half-life. A plot of 1/kobs versus 1/[CHD] gives a k1 rate constant of 6.8 × 10-4 with a k-1/k2 ratio of 3.8. In comparison, the angular 5,6-DEQ shows no rate dependence on 1,4-cyclohexadiene concentration (step 1 is rate determining), though the rate of product formation again lagged behind the rate of starting material disappearance at lower concentrations of 1,4-cyclohexadiene. Overall, these studies confirm our computational data indicating that extended benzannelation orientation can alter enediyne cyclization energetics. Furthermore, the averaged k1 rate constant for the disappearance of 5,6-DEQ (3.0 × 10-3) was measured to be 4-fold faster than that obtained for 6,7-DEQ (6.8 × 10-4) suggesting that the angular quinoxalenediyne has a lower barrier towards Bergman cyclization that may be used to control reaction pathway.
Table 1. Experimental Kinetic Data (180 °C, PhCl).
compound | [CHD] M | kobs sm (s-1) | kobs pdt (s-1) |
5,6-DEQ | 0.28 | 3.37 × 10-3 | 2.87 × 10-3 |
[5.5] mM | 0.41 | 3.04 × 10-3 | 2.99 × 10-3 |
| 0.60 | 2.66 × 10-3 | 2.68 × 10-3 |
|
|
|
|
6,7-DEQ | 0.55 | 8.78 × 10-5 | 8.60 × 10-5 |
[5.3] mM | 0.83 | 1.34 × 10-4 | 1.30 × 10-4 |
| 1.1 | 1.56 × 10-4 | 1.60 × 10-4 |
| 1.3 | 1.71 × 10-4 | 1.74 × 10-4 |
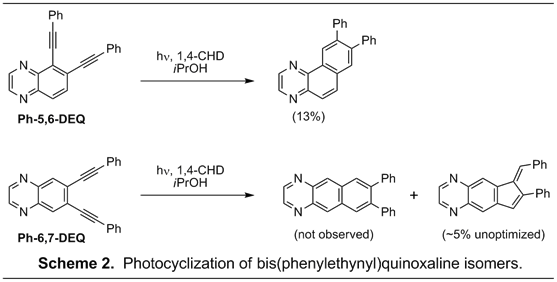
In a second approach to monitor the effect of extended benzannelation orientation on reaction channels of enediynes we synthesized and examined the photochemical reactivity of linear and angular phenylethynyl quinoxalenediynes Ph-5,6-DEQ and Ph-6,7-DEQ (Scheme 2). Here, the angular Ph-5,6-DEQ underwent smooth photochemical Bergman cyclization with generation of the angularly fused azaacene product in 13% yield. Though the yield is not remarkable, it is highly reproducible in the hands of undergraduates and is higher than our yields typically obtained for 1,2-bis(phenylethynyl)benzene (5-10%). In addition, multiple solvent and 1,4-cyclohexadiene adducts are observed by GCMS that we speculate may be forming via Minisci reaction alpha to the ring nitrogen positions. We are currently examining the photoreactivity of the corresponding 2,3-diethyl-5,6-bis(phenylethynyl)quinoxaline derivative in effort to suppress these side reactions and increase the yield of photo-Bergman cyclization. In comparison, the linear Ph-6,7-DEQ shows no evidence of photo-Bergman cyclization products. Instead, initial results indicate the major product is the corresponding C1-C5 cyclization adduct along with formation of the hydrogenated indene product. We are currently optimizing reaction conditions for the linear analog along with its corresponding 2,3-diethyl derivative.
Impact on Undergraduates: During the 2015-2016 academic year a total of six undergraduate students worked on this project with two undergraduate students receiving their degree in spring 2016 (one currently working in industry, one currently in Pharmacy School). In addition, two undergraduate students worked in the Gherman research group in our department conducting computational studies on enediyne derivatives we have prepared in the laboratory. Combined, their work resulted in two presentations at the spring 2016 national ACS meeting and 10 additional campus/regional undergraduate meetings during the academic year. Finally, two undergraduate students conducting this research (one in Spence group, one in Gherman group) participated in our campus McNair’s Scholar Program designed to help prepare underrepresented students for admission to and completion of a Ph.D. program.
Impact on PI's Career: As a PI at a primarily undergraduate institution, conducting quality research with undergraduate chemistry majors is one of my primary responsibilities. In addition to continuing this work with undergraduates, the ACS-PRF grant has facilitated additional funding from various sources on campus including the Academic Senate for release time for scholarly activity, through the McNair’s Scholar Program, and through the Chemistry Department’s Undergraduate Research Scholarship Award that funded an addition summer stipend. This award has also extended my collaboration with Benjamin Gherman (computational chemist in our department) to extend my research program beyond traditional synthetic organic chemistry towards a physical-organic focus.