Reports: DNI153916-DNI1: Regio- and Enantioselective Trifluoromethylation Reactions through a Combined Directing Group/Chiral Trifluoromethylsilane Strategy
Kimberly Petersen, PhD, University of North Carolina at Greensboro
Goals
Due to the increasing importance of fluorine containing molecules, the goal of this proposal is the generation of enantioenriched compounds which contain a CF3 group at a stereocenter. We proposed the accomplishment of this goal using a novel chiral source of nucleophilic CF3 which could be directed to add in a regio- and stereoselective fashion.
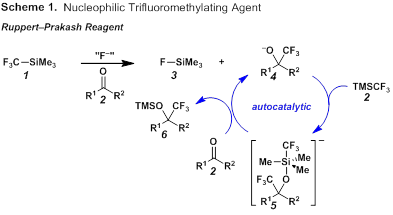
A major challenge to using nucleophilic sources of trifluoromethyl is that the –CF3 anion is unstable and collapses rapidly into a fluoride anion and a stabilized difluorocarbene.1 Because of its commercial availability and easy handling, the Ruppert–Prakash reagent (1 or TMSCF3) employed in combination with an external fluoride source is often the reagent of choice to generate the –CF3 anion.2 A proposed mechanism for the nucleophilic trifluoromethylation of carbonyl compounds is shown in Scheme 1.
The proposal focuses on generation of a chiral silicon based CF3 transfer reagent that would be capable of both 1,2- and 1,4-addition of the CF3 group through a rigid transition state complex.
After several setbacks, the project has shifted somewhat to focus on other methodologies to incorporate a trifluoromethyl group asymmetrically.
Progress
Strained silyacycles
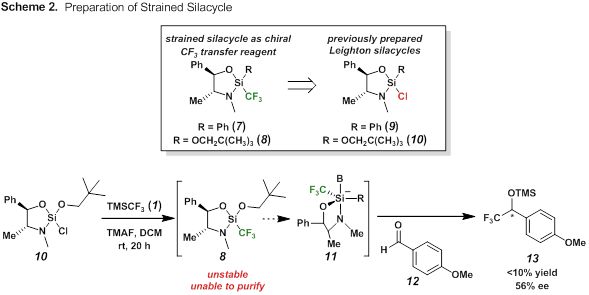
Our initial efforts were on the preparation of a chiral CF3 transfer reagent (7 or 8) based on Leighton's strained silacycles (9 and 10, Scheme 2). Chlorosilanes 9 and 10 were readily prepared as previously described3 and then treated with either (a) phenyl trifluoromethyl sulfone and magnesium or (b) trifluoromethylchlorosilane and an initiator. Unfortunately, while we knew we were generating a new silane (structure 7 or 8?) we have yet been able to purify the product (attempts at distillation were unsuccessful). This challenge led us to explore using the crude material in a nucleophilic addition to test the viability of the reagent. To crude 8 we added the known aldehyde substrate 13 and monitored the reaction for formation of the 1,2-addition product. Encouragingly formation of silyl ether 13 was confirmed (in low yield) and with moderate selectivity (ee = 56%). Regrettably extensive efforts to purify either 7 or 8 led only to decomposition. We spent considerable time working with the crude mixture, but never were able to improve the initial yield.
Silicon Centered Chiral Reagent
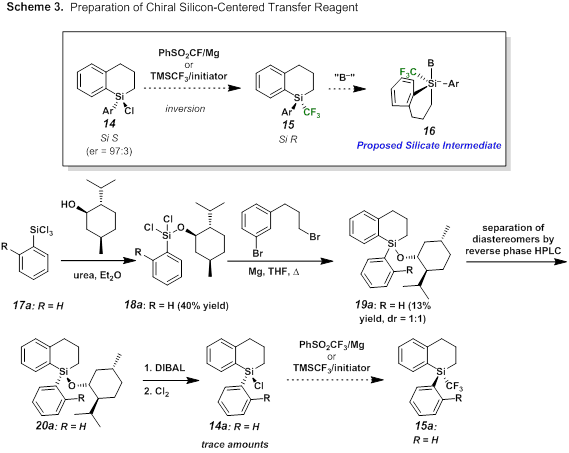
In addition to our work with the strained silacycle 8, we attempted the preparation of proposed CF3 transfer reagent 15 with silicon centered chirality (Scheme 3). Preparation of reagent 15 from known chiral chlorosilane 144 was anticipated to occur with inversion of stereochemistry.5 Treatment with an initiator would then predictably generate silicate 16 which could be used to deliver CF3 in a stereoselective manner. Following Oestreich's protocol, we were able to successfully synthesize and purify a single diastereomer of silane 20a. Reduction and chlorination then led to trace amounts of chlorosilane 14. Due to the undesirable yields of chlorination, we used racemic silanes 14 and 21 as test substrates for incorporation of the trifluoromethyl group (Scheme 4). Unfortunately multiple attempts from either starting material resulted in either recovered starting material or undesired product formation.
Side Projects
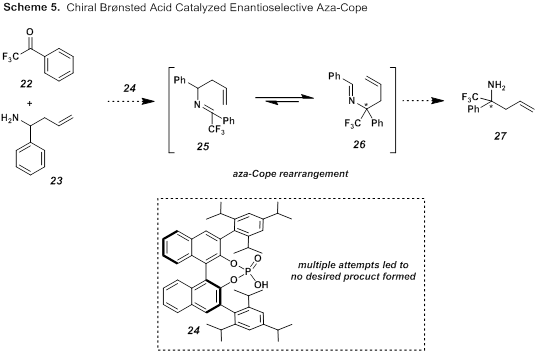
Alongside our attempts to initiate a program based on nucleophilic addition of CF3, we have explored a variety of methods using organocatalysts and readily available trifluoromethyl containing starting materials such as 2,2,2-trifluoroacetophenone 22 to generate enantioenriched small molecules (Scheme 5). One route we examined, was based on Rueping's enantioselective aza-Cope rearrangement using a binol based Brønsted acid catalyst.6 Here, we attempted the reaction of trifluoromethylated ketone 22 with amine 23 in the presence of catalyst 24. Unfortunately, after many attempts we were unable to see any of the desired rearranged products 26 or free amine 27.
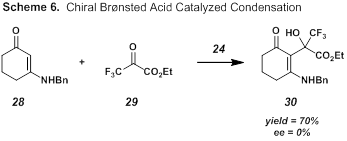
We also explored the condensation reaction of enamine 28 with readily available trifluoropyruvate 29 in the presence of chiral Brønsted acid 24 (Scheme 6).7 While we were able to successfully form product 30 in 70% yield, the product was racemic.
Other
With the close of this grant, our research program is shifting our focus and exploring how we can incorporate other ideas such as Brønsted acid catalyzed desymmetrizations and kinetic resolutions to prepare molecules containing the trifluoromethyl group in an asymmetric manner.
Impact
This grant has had a tremendous impact on my research program. The grant has allowed me to travel to conferences such as the Stereochemistry Gordon Conference in Salve Regina in July of 2016. Undoubtedly, the award was a factor in my laboratory being granted funding from the NIH National (GM116041). My graduate student, Jennifer Wilent, who was supported by this grant was able to focus on research and was very productive this past year with 1 publication published and 2 others in progress. Jennifer graduated this summer (2016) and has taken a job at Weylchem in South Carolina. Additionally, undergraduate researchers who participated in summer research have presented research at conferences and made choices to pursue graduate degrees in chemistry.
References
(1) Langlois, B. R.; Billard, T.; Roussel, S. J. Fluorine Chem. 2005, 126, 173–179.
(2) Prakash, G. K. S.; Yudin, A. K. Chem. Rev. 1997, 97, 757–786.
(3) Kinnaird, J. W. A.; Ng, P. Y.; Kubota, K.; Wang, X.; Leighton, J. L. J. Am. Chem. Soc. 2002, 124, 7920–7921.
(4) Oestreich, M.; Schmid, U. K.; Auer, G.; Keller, M. Synthesis 2003, 2725–2739.
(5) Hathaway, S. J.; Paquette, L. A. J. Org. Chem. 1983, 48, 3351–3353.
(6) Rueping, M.; Antonchick, A. P. Angew. Chem. Int. Ed. 2008, 47, 10090–10093.
(7) Ogawa, S.; Iida, N.; Tokunaga, E.; Shiro, M.; Shibata, N. Chem. Eur. J. 2010, 16, 7090–7095.