Reports: UR755468-UR7: Petroleum-Based Epoxy Mirrors with Optically Smooth Surfaces
K. Lisa Brodhacker, PhD, Lander University
With hopes of increasing the modulus and lowering the coefficient of thermal expansion of our epoxy system, we began an investigating carbon nanotubes which are well-known for their strength and thermal properties. In the fall of 2015 a student researcher (not associated with this grant) started with off-the-shelf multi-walled carbon nanotubes (MWCNTs) and made various samples using our epoxy. Although the data was promising, we did not have adequate dispersion of the nanotubes as seen in figure 1.
Figure 1- Using transmission electron microscopy, we found that the unfunctionalized carbon nanotubes were not dispersed in the polymer.
During the summer of 2016, a student was hired with this grant to continue this investigation. For this study we used MWCNTs functionalized with alcohol groups. These were readily available and reacted easily with the epoxy. The functionalization allowed the carbon nanotubes to be covalently bonded to the polymer removing the challenge of creating uniform dispersion. The task of the student was to find a good procedure (including magnetically stirring and sonication) for incorporating the nanotubes and then make samples with varying amounts of nanotubes (0.5%, 1%, 3% and 5%). We also chose to make samples with the two main epoxy mirror formulations (a 50/50 mix of DGEBA and ED757 and a 100% ED757). Samples were tested for the CTEs and glass transition temperatures using a Thermomechanical Analyzer (TMA) and for dispersion using Transmission Electron Microscopy (TEM). Results show that as the amount of CNTs increase, the CTE decreases and the glass transition temperature increases. We were very excited to see the TEM images which indicate better dispersion than previous research with non-functionalized MWCNTs. (see figure 2).
Figure 2- This micrograph shows much better dispersion of a 3% CNT functionalized with an alcohol group.
Also, work previous to this summer incorporated polyhedral oligomeric silsesquioxanes (POSS) into the epoxy mixture. Incorporating POSS into epoxy/amine polymers combines the advantages of both organics and inorganics to improve the modulus, thermal properties and chemical resistance as well as lower the shrinkage of the polymer. Because the thermal properties were improved in the previous work, we chose to continue investigating this as well.
The summer student also made samples with varying amounts of MWCNTs mixed with various amounts of POSS. For this study we used the same alcohol functionalized MWCNTs in 1% and 3% amounts as well as three variations of POSS: glycidyl POSS with 8 epoxy functionalities, glycidylisobutyl POSS with 1 epoxy functionality, and aminopropylisobutyl POSS with 1 amino functionality. We are currently still testing and analyzing the data from these samples.
Milliken Coprporation donated an Instron to us a few years ago for modulus testing. However, we could not get it working and therefore we could not do modulus testing on site. However, I have been able to make arrangements with Milliken Corporation to use their equipment for testing. Modulus testing from this summer’s research has begun and will be completed next month so we will have the data for our publication in the near future. This link with Milliken is also advantageous for the education of my research students as it gives them time in the chemical industry so they can add that experience to their resumes.
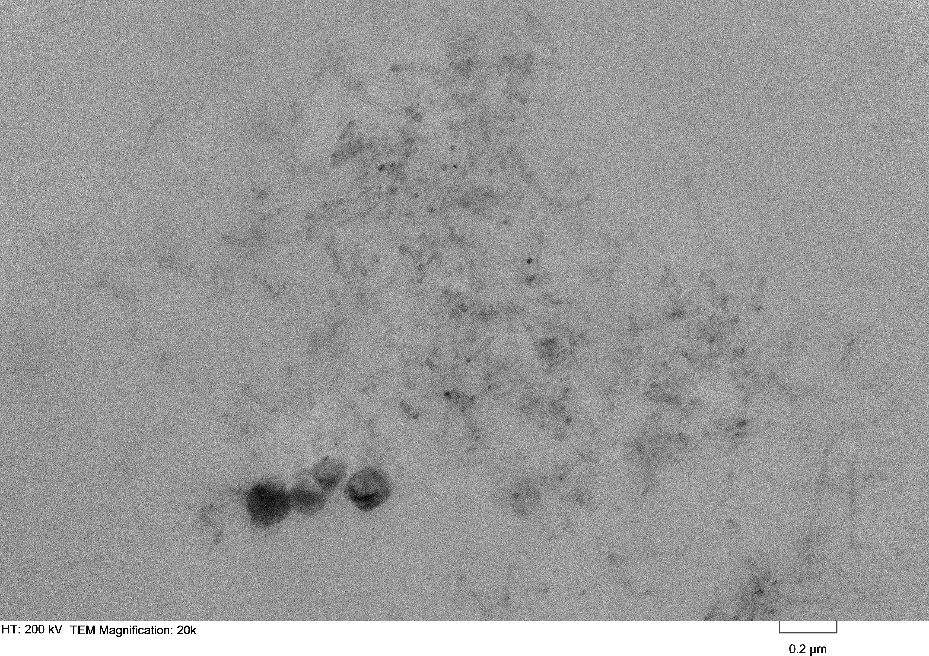
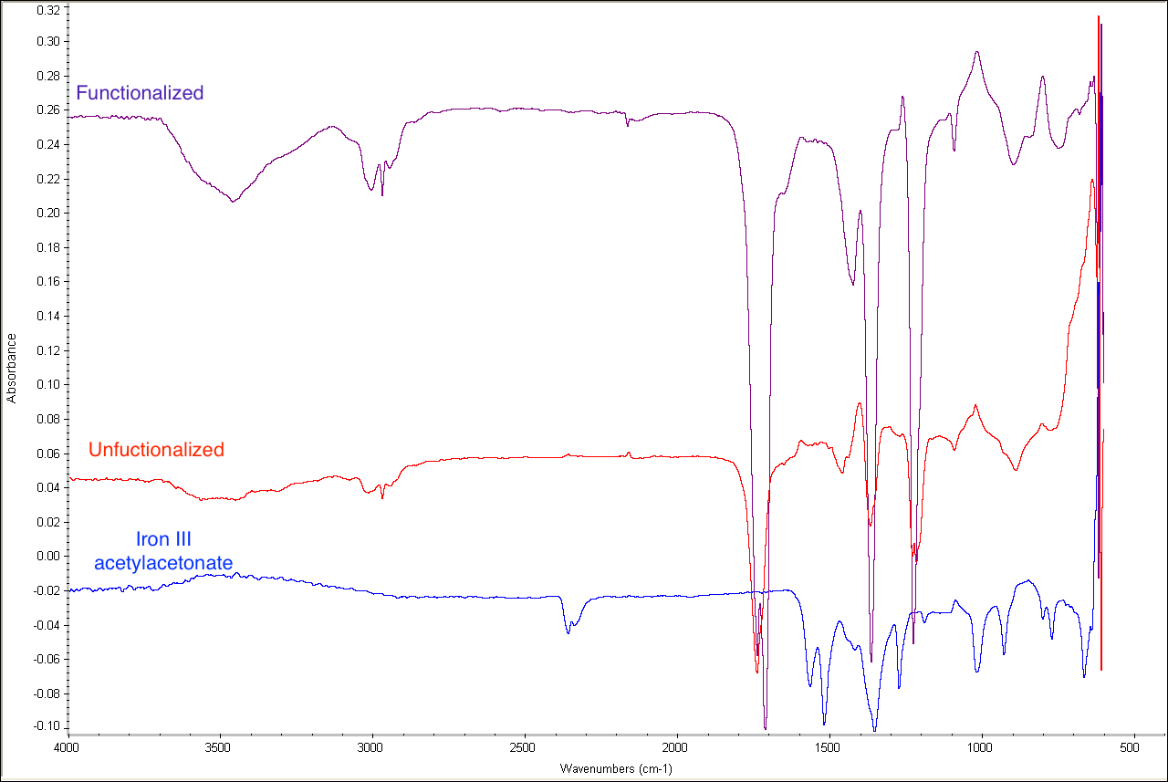
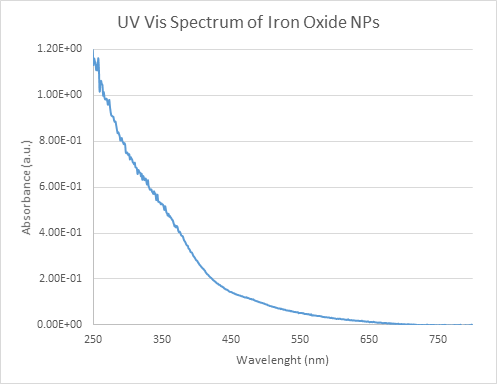
In addition to our work with MWCNTs and POSS, we began to lay the foundation for our shape memory polymer. Previous studies of adding black iron oxide and iron filings to the epoxy shows that we can manipulate the mirror with magnetic actuators on the back. However, just as with non-functionalized MWCNTs, there is inadequate dispersion of these iron particles. To improve this another summer student was hired with this grant to synthesize magnetic iron oxide nanoparticles. The nanoparticles are functionalized and small enough to stay dispersed but we have yet to ascertain if they are strong enough to move the surface of the mirror. Characterization of the nanoparticles was completed through IR and UV-Vis Spectrocopy and Transmission Electron Microscopy (figures 3-5).
Figure 3: IR Spectroscopy confirms the amine functionalization of the iron oxide nanoparticles with the broad N-H peak at 3400 cm-1. The strong peak at 600 cm-1 originates from the Fe-O stretching as seen in the functionalized, unfunctionalized and the starting material (iron III acetylacetonate).
Figure 4: UV-Vis spectroscopy confirmed the synthesized Fe3O4 nanoparticles by showing continuous absorption in the visible range between 200 to 600 nm.
Figure 5: The TEM micrograph of the iron oxide nanoparticles shows monodispersed nanoparticles of 5-10nm in THF.