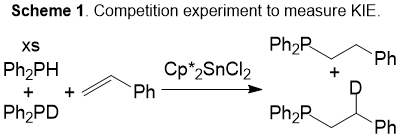Reports: ND354820-ND3: Reductive Elimination at Group 14 Elements: A Pathway Toward Catalysis
Rory Waterman, University of Vermont
I. Research progress.
Efforts under this award have branched in two directions. The main state goal of observing facile reductive elimination from tin(IV) centers is still the main focus of the work. Over the last year, however, we have accrued some evidence that tin(IV) may engage in sigma bond metathesis. This intriguing observation has opened a new avenue of investigation for this project.
I.a. Sigma bond metathesis
The main focus of this projects remains the observation of facile reductive elimination from tin(IV) compounds. In this first project year, we have made some in-road toward that goal. From prior studies, we had been investigating tin(IV) derivatives Cp*2SnCl2 and Ph2SnCl2 as pre-catalysts for phosphine dehydrcoupling and hydrophosphination. In the course of those studies, we identified evidence that these tin(IV) compounds may engage in sigma-bond metathesis. The evidence to suggest this conclusion arises from a competition experiment that provides information about the relative rates of E–H versus E–D bond activation. In that work, hydrophosphination of styrene was performed using an excess of an equivalent mixture of diphenylphosphine and diphenylphosphine-d1. The products were measured, and the all proteo product was favored by approximately three-to-one. This measurement of product give a kinetic isotope effect of 3.1(3) (Scheme 1). This enticingly high value minimally argues for strong P–H bond activation in the turnover limiting step, but it suggests that this step is greatly impacted by specific factors of the transition state. A likely reaction type that gives rise to KIE values of this magnitude is sigma bond metathesis. To the best of our knowledge, data to support such a mechanistic hypothesis has not been reported, and it therefore seemed important to pursue this possibility. Should sigma bond metathesis be available to tin(IV), then early transition-metal type reactivity would be available to a significantly less oxophilic metal, which would be important for energy-related transformations.
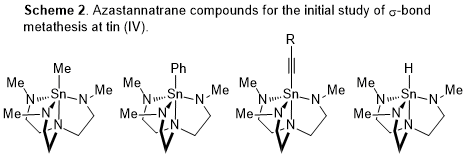
To identify this elementary step, derivatives in which a clear stoichiometric reaction occurs is ideal. Cp*2SnCl2 does not satisfy that criterion because reaction with any E–H bond does not form a single product due to multiple reaction sites. We further were stymied in efforts to accrue better mechanistic evidence to support or refute s-bond metathesis with this system because simple derivatives like Cp*2Sn(PR2)2 were not isolable in pure form, in our hands. These observations argued for simple derivatives that would be amenable to study. As an initial foray, stannatranes are an attractive category of compounds to study. These tin(IV) compounds are readily prepared from inexpensive precursors, examples are well characterized in the literature, and they are of broader utility. We have prepared some of these initial derivatives and are investigating ligand exchange reactions as a venue to identify evidence to prove or disprove such a mechanism (Scheme 2).
II.b. Reductive elimination
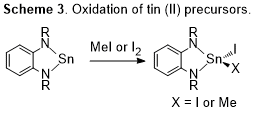
Progress toward identifying facile reductive elimination from Sn(IV) has been proceeding forward. As described we have been preparing selected tin(II) (i.e., stannylene) compounds that we have been oxidizing to tin(IV). Surprisingly the derivatives selected were more challenging to prepare than anticipated. The oxidation of these compounds was not reported, and reactions with methyl iodide or iodine resulted in the formation of the expected tin(IV) products. Precise control of stoichiometry was unexpectedly critical for both reagents. One might anticipate that an excess of methyl iodide may lead to alkylation of nitrogen at the ligand. However, even trivial excess of iodine (e.g., 0.02%) lead to significant mixtures of products. We have devised reliable preparations of tin(IV) compounds and have been proceeding to make derivatives to test the proposed hypotheses (Scheme 3). Simple reversibility of the oxidation reactions has not been observed, even in the presence of traps for iodine and methyl iodine. This is an expected observation, but it is also an important control.
In sum, this initial year has been highly successful, and we are well poised to collect critical data in the coming year that will launch this into a sustainable, long-term project.