Reports: DNI1055708-DNI10: Syntheses of Polycarbonyl Aromatic Compounds for New Redox Materials
Xiulei Ji, PhD, Oregon State University
We have demonstrated, for the first time, a polycyclic aromatic hydrocarbon (PAH) - crystalline and readily available coronae - exhibits highly reversible anion-storage properties. Conventional graphite anion insertion electrodes operate at potentials > 4.5 V vs Li+/Li, requiring additives and the use of ionic liquids. The coronene electrode shows flat plateaus at 4.2 V (charge) and 4.0 V (discharge) in a standard alkyl carbonate electrolyte and delivers a reversible discharge capacity of ~ 40 mA h g-1. The discovery of the reversible anion-storage properties of coronene may open new avenues toward dual-ion batteries based on PAHs as electrodes.
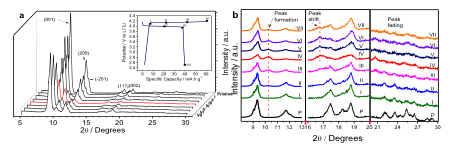
Figure 1a inset shows the first-cycle charge/discharge potential profiles of the coronene electrode in electrolyte of LiPF6 (1.0 M) solvated in ethylene carbonate (EC) and diethyl carbonate (DEC) (v/v 1:1). Furthermore, to better understand PF6- incorporation into the coronene electrodes, we investigated the evolution of crystal structure and chemical bonding as a function of the state of charge. Figure 1a inset shows the selected points of state of charge at which ex situ measurements were performed (Figure 1a,b).

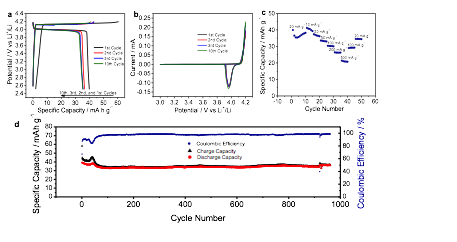
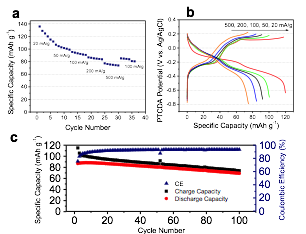
Figure 2a shows the charge/discharge profiles for the 1st, 2nd, 3rd, and 10th cycles, where the plateau behavior is well maintained. Cyclic voltammetry (CV) results show high reversibility in the first 10 cycles (Figure 4b). Figure 2c shows the rate capability, where coronene is able to deliver ~21 mA h g-1, even at a high current density of 500 mA g-1, despite its low conductivity. The coronene electrode exhibits impressive long-term cyclability, where after 960+ cycles, the capacity retention is 92% (Figure 2d). Furthermore, coulombic efficiency in the long-term cycling study rises from 67.1% in the 1st cycle to 97.3% in the 100th cycle, 98.5% in the 300th cycle, and 98.6% in the 900th cycle.

Scanning electron microscopy (SEM) studies were conducted on coronene electrodes before and after insertion of PF6- anions (Figure 3). The macro-scale structural changes of coronene are evident, where the rod-like crystals of over 5 µm long and ~3 µm wide (Figure 3a) crack upon the first charge (Figure 3b) and turn into porous crystals after 400 cycles (Figure 3c). This explains why the capacity only drops in the first 50 cycles and is stable henceforth. The surprisingly stable cycling of coronene may be associated with the formation of the more porous structure with a reduced strain for PF6- anion insertion.


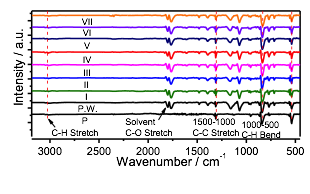
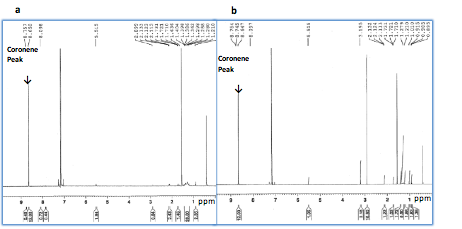
Ex situ nuclear magnetic resonance (NMR) performed upon full charge confirms the absence of new bonding in coronene (Figure 4). Ex situ Fourier-Transform Infrared (FTIR) spectra were also used to characterize changes in chemical bonding in coronene upon PF6- incorporation (Figure 5). Excluding electrolyte peaks, it is clear that no new peaks arise upon charging, suggesting that new chemical bonds formed are not formed during charging.
Furthermore, we, for the first time, report that 3,4,9,10-perylenetetracaboxilicdianhydride (PTCDA), exhibits high rate performance in Mg-ion storage and stable cycling, as shown in Figure 6.
 |