Reports: UR453159-UR4: Impact of Solvent-Solute Interactions on Photochemistry of p-Aminobenzoic Acid Derivatives
Sarah J. Schmidtke Sobeck, PhD, College of Wooster
Our research is focused upon the impact of environment and structure on the physico-chemical properties of para-aminobenzoic acid (PABA) derivatives. A general scheme for the potential photo-induced intramolecular charge transfer (ICT) is shown in Figure 1. During the first year of ACS-PRF funding we assessed the solvent-dependence of the ICT process and its thermodynamics when present. Last year photo-stability studies of PABA and an ester derivative commonly used in sunscreens were initiated. Additionally, we carried out synthetic work modifying the electron acceptor moiety at the R2 and R3 positions. In the present year our work has focused upon optimizing the synthesis, and evaluating the solvent-dependence of the photodegradation of PABA and its derivatives.
Figure 1. Potential ICT reaction from the locally excited (LE) to zwitterionic (ZI) states for PABA derivatives.
Results
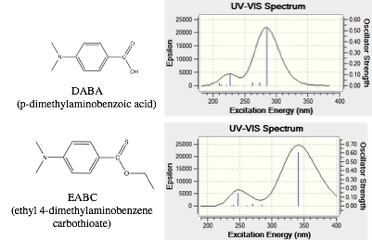
In the 2015-16 academic year three undergraduate students were engaged in research related to the PABA projects. Haley Rossiter continued her synthetic work, describe in the 2015 narrative, optimizing synthesis modifying the electron acceptor moiety. She was able to synthesis and characterize a series of five derivatives. Her characterization consisted of IR, NMR, UV-Vis absorbance, and fluorescence. Thionation, at the R2 position, results in a loss of fluorescence and a red-shift in the UV-absorbance. Computational modeling support a decrease in the HOMO to LUMO gap (Figure 2).
Figure 2. Structures and calculated absorbance spectra for DABA and a thionated ester derivative.
Two seniors carried out photodegradation studies of PABA compounds for their Independent Study work. Preetom Borah continued his studies from the summer comparing the photo-stability of PABA with Padimate-O, an ester derivate of PABA commonly used as a UV-absorber in personal care products. Padimate-O is able to undergo charge transfer leading to more efficient non-radiative decay and a lower quantum yield. Both compounds have a nearly complete degradation over a 24-hour UV exposure and the degradation rate constant is dependent upon the solvent environment.
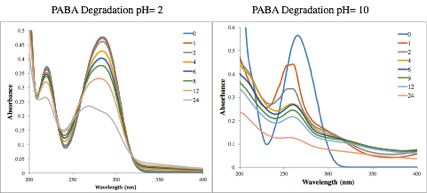
Briana Marlatt assessed the impact of pH and ambient environment upon the degradation of PABA. A (slow) unimodal process if followed under acidic conditions, but there is a bimodal decay under basics conditions (Figure 3). The fast kinetics, observed at high pH, did not show an oxygen dependence. In contrast the slower decay, observed in all solutions, were first-order kinetics and rates increased in the presence of oxygen. Initial work was carried out to test if antioxidants may stabilize the compounds.
Figure 3. Comparison of the degradation of PABA in acidic versus basic aqueous buffer solutions, followed using UV-absorbance, over the course of a 24 hour UV-exposure. Time at which the spectra were acquired are in hours.
This summer a rising sophomore student, Gabriela Jocas, continued the UV-degradation studies comparing the solvent and ambient environment dependence of the degradation of PABA and DABA (dimethyl-aminobenzoic acid). She is continuing this work in the present academic year. A second student, Madeline Thomas, began work on evaluating methods for following degradation of solid-samples as we begin work with films. She initially developed a method to use our solid state mount and evaluate the best angle / instrument settings to measure a reflectance spectrum using our fluorimeter. To understand how kinetic rate constants compare depending upon the spectral monitoring used, she ran solute-phase degradation trials monitoring both absorbance and emission spectra throughout the irradiation. We found that fluorescence spectra do provide timescales that reflect those measured by absorbance, but there is a larger error between trials.
Student Impact
During this last academic year four of my research students, including Briana, Haley, and Preetom, presented at the National American Chemical Society Meeting in San Diego. Madeline and Gabriela presented their work on campus, at poster sessions, during our fall research showcase and at family weekend. During this summer, my two summer students also assisted in leading an evening activity at the Buckeye Women in Science and Engineering (B-WISER) camp, a camp for 7th and 8th grade girls in Ohio to expose them to different STEM fields with hands-on activities.
Following graduation, Preetom participated in a NIST SURF program in Maryland and he is currently applying for graduate programs in material's engineering. Briana Marlatt is working as a chemist in a lab focused upon personal care product formulation. Haley Rossiter participated in a REU program at the University of Southern California this summer and is now starting her senior year at Wooster. Her Senior Independent Study project builds upon the foundation laid out by her prior work on the fundamental origin of the PABA derivatives' physicochemical properties.
Ongoing Plans
During the present academic year Gabriela Jocas is continuing research in my lab. Additionally, the I am advising four Senior Thesis students. Haley Rossiter's work has evolved to focus on the physicochemical properties different aminobenzoic acid derivatives. She will be assessing the physicochemical properties of a series of aminobenzoic acids with variations in the substituent positions (ortho-, meta-, and para-). Two other students, including Madeline, are studying the photochemistry of dyes and pigments focusing upon organic (anthraquinone) dyes and pigments using in cultural heritage and in foods.
I am currently finalizing a manuscript, with an intended submission this fall, on the optimized synthesis and characterization of the thiol and halogenated derivatives. This will include several student co-authors, including Haley. We are also compiling the degradation studies of the PABA species to present at the winter I-APS (Inter-American Photochemical Society) conference and future submission as a full article. This last year we did publish our initial paper on the carmine degradation, based in part upon work carried out during and following my research leave during the 2014-15 academic year.
The PI and her group continue to appreciate the generous support from the ACS PRF grant. This support makes the full-time (immersive) summer research experience possible, which is so meaningful to the scientific development of our students.