Reports: DNI654407-DNI6: Direct Visualization of Interfacial Energy-Transfer and Charge-Transfer Dynamics of Thin Ionic Liquid Films by Ultrafast Electron Imaging
Ding-Shyue Yang, PhD, University of Houston
Project Objectives
The goal of this research is to understand ultrafast dynamics at the interfaces between solids and room-temperature ionic liquids (ILs). Previously, time-resolved all-optical spectroscopic methods were used to examine photoinduced dynamics in homogeneous liquids and solutions. For heterogeneous phases, reports about the equilibrium interfacial orders of ILs now attract more attention. However, corresponding dynamics investigations have been largely absent to date, except for the limited initial studies of electron hydration at a metal-IL interface using two-photon photoemission spectroscopy. We intend to fill this knowledge gap using time-resolved electron diffraction, which has surface specificity and the advantage of direct probing of assembly structures.
In the first year, we have successfully demonstrated the method of reflection high-energy electron diffraction (RHEED) as a contactless, surface-specific method to probe the ion organization and layering at solid-IL interfaces. More studies of the equilibrium structures are required before examining the influence of the orders of ILs and interfacial interactions on the dynamics of energy transfer, charge transport, and/or electron solvation processes. In addition, we are interested in the behaviors of other molecular species that also have strong intermolecular forces; a comparison with the results of interfacial ILs may provide further information about the unique roles and impacts of Coulombic interactions between ions.
In the second year, we investigated interfacial methanol on smooth hydrophobic silicon substrates. Using RHEED, unexpected observations of 2- and 3-dimensional ordered assemblies of methanol molecules were found for the first time. Even without much guidance from the smooth substrate, interfacial methanol undergoes structural transformations beyond the commonly presumed single-stage description, which strongly depend on the thermal annealing procedures used. These results illustrate the importance and opportunity of direct structure-probing of interfacial phenomena.
Results and Discussion
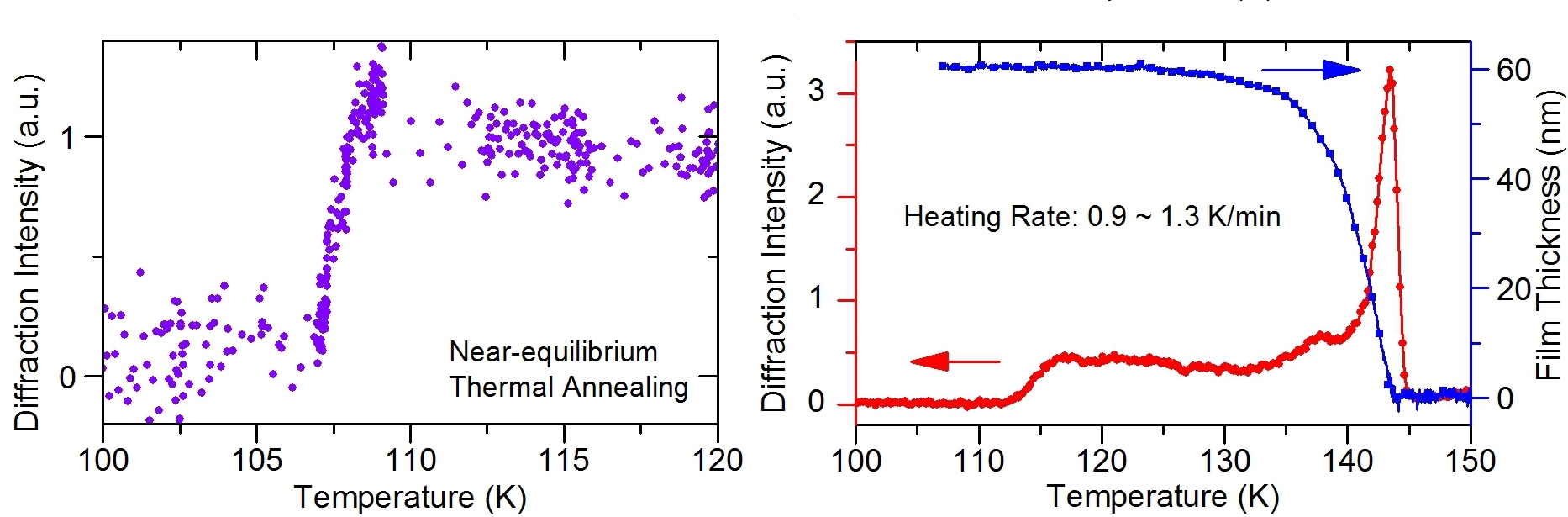
Previously, vapor-deposited (ultra)thin films of methanol on cold solid substrates were investigated using calorimetry, temperature-programmed desorption, optical reflectance, ion scattering, and infrared spectroscopy methods. The structures of multilayer assemblies were indirectly inferred based on interpretation of the temperature-dependent changes in the observables. However, inconsistent results were reported even for basic properties such as the glass-transition and crystallization temperatures (Tg and Tc, respectively), whose experimental distinction was often obscure. Compared to Tg = 103 K and Tc = 105 K for bulk methanol, the value of Tc for solid-supported films was reported to be 108 K, in the range of 112‒116 K, around 130 K, or as high as 145 K if a metastable phase exists. Even if the rate of thermal annealing and the vacuum condition are taken into consideration, it is still surprising to find a large disparity in Tc for a simple and abundant molecule like methanol.
Figure 1 shows the striking differences between the structural transformations of interfacial methanol and those of interfacial water. Both vapor-deposited molecular films at 90 K yield a diffuse scattering pattern, where amorphous solid water (ASW) exhibits more notable ring-like features that are associated with a disordered but locally hydrogen-bonded assembly. Upon thermally annealing above Tc, randomly oriented crystallites of the cubic structure grow in thin ASW and hence a pattern of Debye-Scherrer ring diffractions is seen.
In contrast, crystallization of amorphous solid methanol yields a pattern of streaks and then that of sharp Bragg spots at higher temperatures. Such diffraction features reveal an unexpected orientational order, instead of a polycrystalline assembly, for interfacial methanol on topographically smooth, hydrophobic hydrogen-terminated Si(111). According to the principles of RHEED, a streaky pattern at grazing incidence angles signifies an atomically smooth (or, at most, weakly stepped) 2D-ordered film surface, whereas transmission-like Bragg diffractions mean the presence of nm-sized 3D crystals, often as a result of single-crystalline materials with larger surface roughness. Thus, the present observations attest the rich structural transformations of interfacial methanol beyond the description of single-stage crystallization. We obtained the lattice constants of a = 4.71 Å, b = 4.93 Å, and c = 9.13 Å at 145 K, which match well with the bulk values determined using a single crystal at 122 K.
The diffraction results show that chains of hydrogen-bonded methanol molecules surprisingly grow only in the horizontal direction even if they are tens or hundreds of nanometer away from the substrate surface. This indicates the strong tendency of self-assembling for interfacial methanol. At lower temperatures, nevertheless, only horizontal bilayer networks are formed readily, resulting in an atomically smooth, ordered film surface that gives a streaky diffraction pattern. The development of the crystalline order in the vertical direction takes place at higher temperature.
Figure 2 shows the diffraction intensities as a function of temperature for different thermal annealing procedures. It was found that the center (00) diffraction streak appears and reaches its intensity plateau at 107‒108 K if each target temperature is maintained within a 1-K range for 10 minutes or more. At a heating rate of ~1 K/min, the (00) diffraction streak becomes apparent at a higher temperature range of 112‒116 K instead. Such a temperature shift may be anticipated for slow nucleation-related phenomena during a relative fast thermal annealing process, and our results provide a framework to unify previous observations. However, the preference to form hydrogen bonds exclusively along the surface directions, which results in the anisotropic (compared to the case of interfacial water ice), 2D horizontal order, is unexpected and uncovered only now via direct structure probing.
We also confirmed that 3-dimensionally ordered interfacial methanol is formed as a result of molecular desorption from the substrate surface. When the substrate temperature is further elevated above ~130 K, the film thickness decreases more quickly and the transmission-like Bragg spots become more prominent. A striking resemblance has been found in the temperature dependence of the sublimation rate and that of the crystal growth rate.
Impact and Future Plans
The discoveries thus far illustrate the different behaviors of molecules and ILs in a heterogeneous environment. Currently, we are conducting dynamics studies of these interfacial systems as well as static studies of other solid‒molecule structures. Graduate students supported by the ACS PRF grant acquired direct experiences working with ILs, thin-film depositions, and advanced experimental techniques.