Reports: DNI1055570-DNI10: A Novel Microtubular Mesoporous Carbon Catalyst Support
Jiahua Zhu, PhD, The University of Akron
PROGRESS REPORT
With the support of ACS PRF, significant research progress has been made over the past year focusing on the topic of biomass processing into functional materials including but not limited to catalyst support. By completing the first-year goal, additional efforts were devoted to explore other related research areas as well. The research progress is summarized as follow.
(1) Processing biomass into hierarchically porous carbon catalyst support
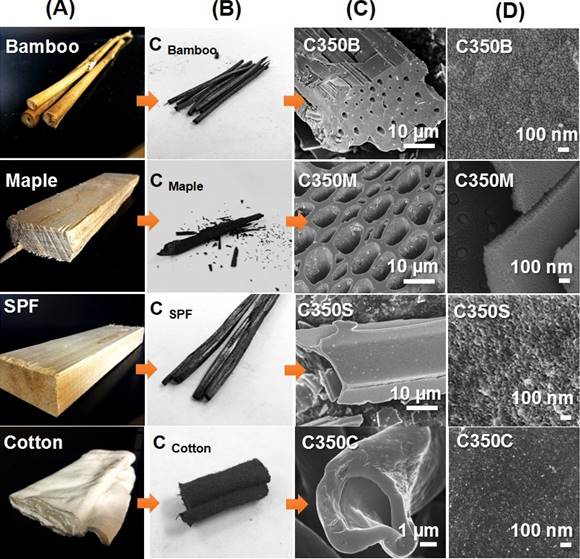
Figure 1. (A) Photos of raw plant biomass-bamboo, maple, spruce-pine-fir (SPF) and cotton, (B) carbonized biomass, (C) SEM microstructure of carbonized biomass after oxidation at 350 oC and (D) their porous surface morphology.
Four different plant biomass, bamboo, cotton, soft wood and hard wood, were utilized as carbon precursors to fabricate porous carbon catalyst supports via a chemical free approach, Figure 1. Large surface area with unique mesoporous structure was successfully created in the carbon, which made them suitable for catalyst support. After decorating silver nanoparticles onto these carbon supports, nitroaromatics reduction reactions were performed to evaluate the catalyst activity. Results indicate that chemical composition and surface groups of carbon supports determine the metal catalyst nucleation/growth while the porous microstructure of support affects the mass transport of reactant/product across the liquid/catalyst interface. Among the four selected biomass, porous carbon manufactured from SPF acquires the highest average pore size, pore volume, mesopore volume fraction and best catalytic activity after decorating silver nanoparticles. This study provides a facile and sustainable approach to convert biomass into catalyst support and offers a comprehensive understanding of unique biomass structure and composition relating to their suitability for catalyst support development.
(2) Catalyst deposition onto biomass derived porous carbon: Alcohol effect
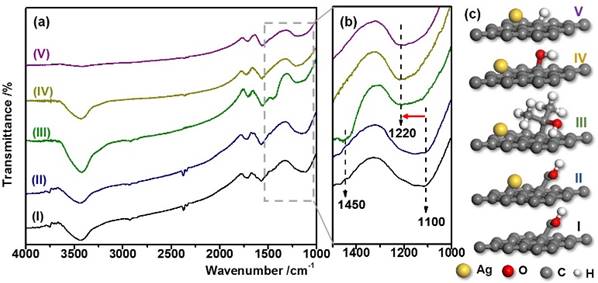
Figure 2. FT-IR spectrum of (a-I) oxygenated mesoporous carbon (OMC), (a-II) Ag/OMC-Ethanol, (a-III) Ag/OMC-Methanol, (a-IV) Ag/OMC-Propanol, (a-V) Ag/OMC-H2O, (b) enlarge spectrum in the range of 1000-1500 cm-1, (c) Schematic illustration of alcohols effects on substrate surface.
Alcohols (methanol, ethanol and isopropanol) have been found to affect the heterogeneous nucleation and growth of Ag nanoparticles onto oxygenated mesoporous carbon support and the corresponding catalytic property of Ag/Carbon nanocomposite in nitroaromatics (4-nitrophenol and 2-nitroaniline) reduction reactions. Ethanol exhibits unique capability to regenerate reactive -CH2OH groups on carbon surface and successfully control Ag particle size through extending the nucleation process, Figure 2(a-II). Catalyst prepared with ethanol shows well-controlled particle size and dispersion and thus the highest catalytic activity in reduction reactions. Other alcohols experienced structural rearrangement typically from primary and secondary alcohols to tertiary and aromatic alcohols, which reduced the reactive sites on carbon surface, Figure 2(a-III, a-IV and a-V). The detailed transition mechanism of surface alcohol groups is currently under investigation.
(3) Processing biomass into high surface area porous carbon adsorbent
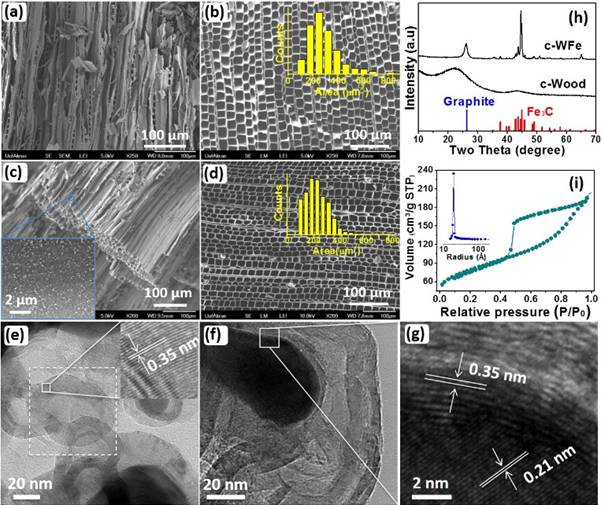
Figure 3. SEM of carbonized wood (c-Wood): (a) side view, (b) top view, inset shows the statistical distribution of pore area. Carbonized wood/iron composites (c-WFe): (c) side view, inset figure shows the nanoparticle distribution on the side wall, (d) top-view and pore area distribution of c-WFe at cross-section area. HRTEM of c-WFe focusing on (e) carbon-inset figure clearly indicates the graphitized carbon with lattice fringe of 0.35 nm, (f) NPs/carbon interface, and (g) enlarged interface of (f) clearly shows lattice fringes of iron carbide (0.21 nm) and graphitized carbon (0.35 nm). (h) XRD pattern of c-Wood and c-WFe. (i) Nitrogen isotherm of c-WFe at 77.4 K and the inset figure shows its pore size distribution.
Mesoporous carbon with embedded iron carbide nanoparticles was successfully synthesized via
a facile impregnation–carbonization method. A green biomass resource, cotton fabric, was used as a carbon precursor and an iron precursor was implanted to create mesopores through a catalytic graphitization reaction. The pore structure of the nanocomposites can be tuned by adjusting the iron precursor loadings and the embedded iron carbide nanoparticles serve as an active component for magnetic separation after adsorption. The microstructure of the nanocomposites was carefully investigated by various characterization techniques including electron microscopy, X-ray diffraction, surface analyzer, magnetic property analyzer and etc, Figure 3. Large surface area of >300 m2/g can be obtained. The newly created mesopores are demonstrated as a critical component to enhance the adsorption capacity of organic dyes and embedded iron carbide nanoparticles are responsible for the selective removal of heavy metal ions (Zn2+, Cu2+, Ni2+, Cr6+ and Pb2+). Isotherm adsorption, kinetic study at three different temperatures (25, 45 and 65 oC) and cycling retention tests were performed to understand the adsorptive behavior of the nanocomposites with organic dyes (methylene blue and methyl orange). Together with the preferable removal of more toxic heavy metal species (Cr6+ and Pb2+), these mesoporous nanocomposites show promising applications in organic pollutant removal from water. The unique dual function towards both organic dye and heavy metal adsorption allows this mesoporous carbon/iron carbide nanocomposites promising applications in practical polluted water treatment not only because the effective adsorption property but also the widely accessible raw material, easy fabrication, low cost and minimum environmental impacts
(4) Impacts
This fund has been used to support one Master student and one more student can be supported with this fund in the next year. By working on this project, the student received tremendous training on literature review, experimental design, materials fabrication, catalytic reaction operation, data analysis and scientific paper writing. More importantly, the gained knowledge, learned lab skills and published work all prepare the student for a future professional career. To the PI, this is the first external fund in his career. Besides the fully completion of the proposed tasks, this fund allows PI to explore new opportunities in related research areas and generate preliminary results for federal grants. Based on the preliminary results, the PI has developed a NSF proposal on the topic of sustainable lubricants by using green biomass as lubricating additives.