Reports: DNI655672-DNI6: Chemical Kinetics of Gasoline Mixtures under Negative Valve Overlap Reforming Conditions
Subith Vasu, PhD, University of Central Florida
Motivation:
Recent efforts have pursued the combustion and performance advantages of in-cylinder reforming processes for negative valve overlap (NVO) controlled homogeneous charge compression ignition (HCCI). In NVO operation a direct injection (DI) fueling system is used to control combustion by timing the fuel injection into NVO operation. When fuel is injected into O2- deficient engine operating conditions, a portion of the fuel can be converted to products containing significant levels of H2, CO, and small chain partially oxidized hydrocarbons (e.g., CH4). Other strategies known as spark-assisted HCCI (SA-HCCI) and spark assisted compression ignition (SACI) have been pursued as well. Fuel chemistry is crucial to the operation of advanced concepts like HCCI and SA-HCCI. However, latest engine measurements revealed the disagreement between data and kinetic predictions for reformate concentrations. Fuels breakdown into intermediates via pyrolysis and oxidation channels, thus the formation and consumption time-scales of intermediates play crucial role in heat release and combustion. Chemical kinetics investigation of gasoline-relevant fuels and intermediates are carried out in this study using two complementary state-of-the-art experimental strategies.
Ignition Delay Times and Species Time-Histories using Shock Tube and Absorption Diagnostics:
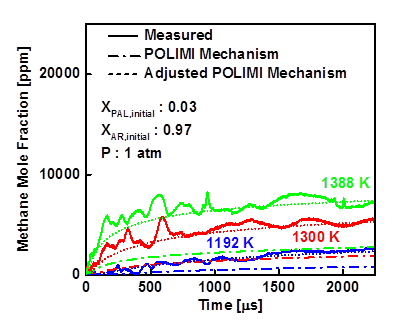
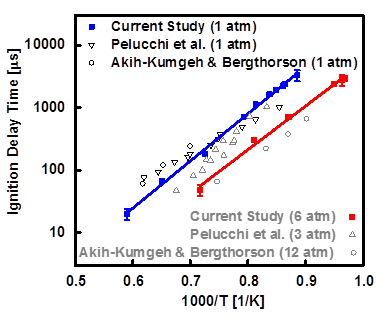
Propanal is an aldehyde intermediate formed during hydrocarbon combustion process. In this work, ignition delay times measured behind reflected shock waves for stoichiometric mixtures of propanal (CH3CH2CHO) and oxygen (O2) in argon bath gas at temperatures of 1129 K < T < 1696 K and pressures around 1 and 6 atm. Measurements were conducted using the kinetics shock tube facility at University of Central Florida (UCF). The results were compared to available data in the literature as well as the predictions of three propanal combustion kinetic models: POLIMI, NUIG, and McGill mechanisms. In addition, we used cw laser absorption diagnostics for measuring methane (CH4) and propanal time-histories behind the reflected shock waves using a continuous wave distributed feedback interband cascade laser centered at 3403.4 nm. Methane and propanal concentration time-histories were obtained during pyrolysis of propanal at temperatures between 1192 K and 1388 K near 1 atm. Sensitivity analysis was carried out to understand important reactions that were crucial to modeling current experimental results. Results from UCF shock tube study are shown in Figures 1-6.
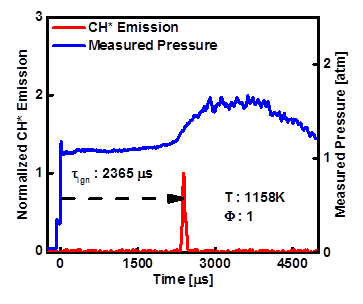
Figure 1- Pressure and normalized CH* emission, 1% propanal in O2/Ar (P5 ~ 1.0 atm, T5=1158 K).
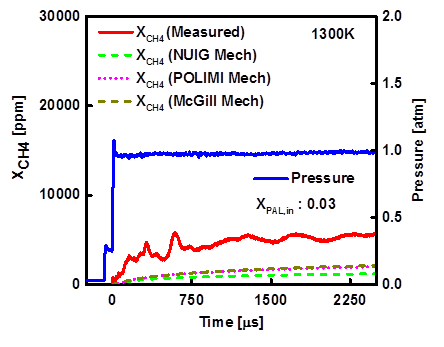
Figure 2- Measured methane mole fraction and pressure time-histories, 1300 K and 1 atm during 3% propanal pyrolysis in argon.
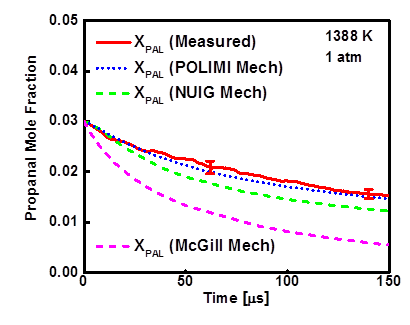
Figure 3- The measured propanal mole fraction time- histories at 1388 K and 1 atm during 3% propanal pyrolysis in argon.
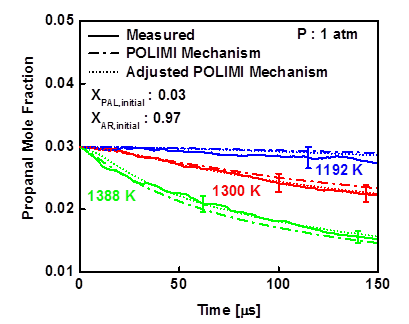
Figure 4- Experimental concentration time-histories for propanal at three temperatures 1192 K, 1300 K, and 1388 K around 1 atm.
Figure 5- Experimental concentration time-histories for methane at three temperatures 1192 K, 1300 K, and 1388 K around 1 atm (during propanal pyrolysis).
Figure 6- Propanal ignition delay times at pressures around 1, 3, 6, and 12 atm.
Products and pathways of aldehydes oxidation using synchrotron PIMS:
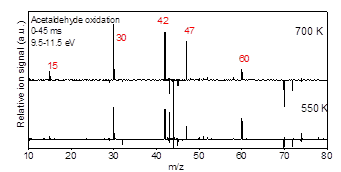
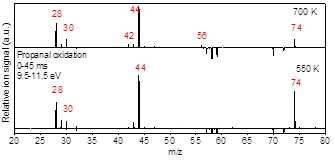
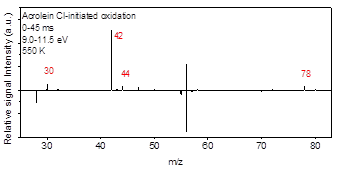
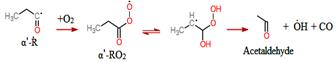
Aldehydes are major intermediates in oxidation and pyrolysis of hydrocarbons and particularly biofuels. While the high temperature oxidation chemistry of C3-C5 aldehydes have been studied in the literature, a comprehensive low temperature kinetics remains unaddressed. In this work, acetaldehyde, propanal, and 2-propenal (acrolein) oxidation was investigated at low-temperature combustion condition (500-700 K). The isomer specific products concentrations as well as the time-resolved profiles were studied using Sandia’s multiplexed photoionization mass spectroscopy (MPIMS) with synchrotron radiation from the Advanced Light Source (ALS). The laser pulsed photolysis generates chlorine atoms which react with aldehydes to form the parent radicals. In the presence of excess oxygen, these radicals react with O2 and form RO2 radicals. The temperature dependent products yields are determined for 500 K to 700 K and the competition between the channels contributing to the formation of each product is discussed. In acetaldehyde oxidation, the formation of the main products are associated with HO2 elimination channel from QOOH or direct H atom elimination from the parent radicals. In propanal oxidation, the most intensive signal peak was associated with acetaldehyde (m/z=44) which was formed through the reaction of alphaꞌ-R with O2. In 2-propenal oxidation, the unsaturated radical produced from alpha-R reacts with O2 to form the primary products. Results from the ALS study are shown in Figures 7-10.
Figure 7- Integrated difference mass spectra for Cl-initiated oxidation of Acetaldehyde at 550 K and 700 K, photon energy 9.5-11.5 eV, and kinetic times until 25 ms after the laser photolysis.
Figure 8- Integrated difference mass spectra for Cl-initiated oxidation of Propanal at 550 K and 700 K, photon energy 9.5-11.5 eV, kinetic times until 25 ms after the laser photolysis.
Figure 9- Integrated difference mass spectra for Cl-initiated oxidation of acrolein (conditions same as propanal).
Figure 10- Acetaldehyde formation pathway from propanal oxidation
Impact of Research and ACS PRF Funding:
We provided the first high temperature species concentration time-histories during propanal pyrolysis and improvements to propanal kinetic mechanisms were suggested. The ALS work addresses the shortage of experimental data on low temperature (500-700 K) aldehydes oxidation pathways and products in the literature. Current data will serve as the first step for developing a comprehensive low temperature submechanism for these major aldehyde compounds. The detailed interpretation of the experimental data will also serve as validation targets for quantum chemical calculations of the potential energy surfaces for these important reaction systems.
The ACS PRF DNI grant has had very significant impact on the PI and both graduate and undergraduate students. The PI was able to recruit and train students for research, as well as publish several journal and conference articles based on this work. These publications will provide professional recognition for the PI while establishing his career. The grant partially supported 3 Ph.D. students to complete their thesis and introduced several undergraduate students to research (some of them are continuing for graduate studies with the PI). Also, the broader impact of the grant is that it helped several underrepresented students to pursue education and research.