Reports: ND654607-ND6: Non-Equilibrium, Atmospheric-Pressure Microplasmas for Hydrocarbon Gas Conversion
R. Mohan Sankaran, PhD, Case Western Reserve University
Daniel J. Lacks, Case Western Reserve University
1. Introduction
The goal of this ACS PRF ND project is to develop an atmospheric-pressure gas discharge (plasma) for non-equilibrium, non-oxidative conversion of methane (CH4) to hydrogen (H2), acetylene (C2H2), and other higher order hydrocarbon gases.
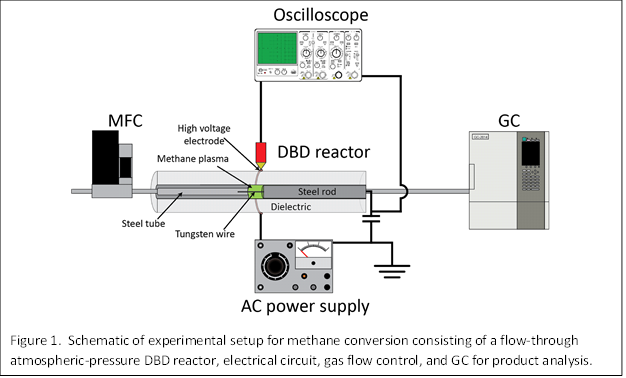
2. Experimental details
The same experimental setup that was built last year was the focus of our studies this past year. Briefly, as schematically illustrated in Figure 1, the setup consisted of a flow-through atmospheric-pressure dielectric barrier discharge (DBD) reactor operated by a high-voltage alternating current (AC) power supply, a digital oscilloscope to monitor the current and voltage waveforms, a mass flow controller (MFC) to supply a desired steady-state flow of CH4, and a gas chromatograph (GC) equipped with thermal conductivity detector (TCD) and flame ionization detectors (FID) to characterize the gas effluent leaving the plasma reactor. A key modification that was made this year was that the plasma was physically confined by adding two electrically grounded metal pieces inside the quartz reactor. As we detail in the results section, the confinement was critical to keeping the plasma volume constant and decoupling the effect of power on reaction conversion and selectivity.
Methane conversion was obtained by relating the peak areas from gas chromatograms to molar flow rate. The fractional conversion, f, was defined as
where ![]() is
the molar flow rate and the selectivity of a species, Si, was
defined as
is
the molar flow rate and the selectivity of a species, Si, was
defined as
3. Results
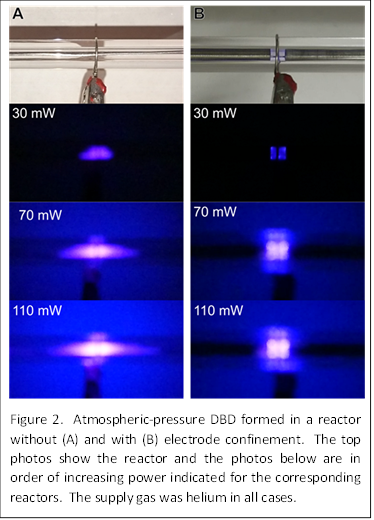
Figure 2 shows a side-by-side photo comparison of the DBD formed with (Figures 2A, right) and without (Figures 2B, left) confinement. Without confinement, the plasma expands to the left and to the right of the electrode as the power is increased. In comparison, the plasma volume remains constant as the power is increased with confinement.
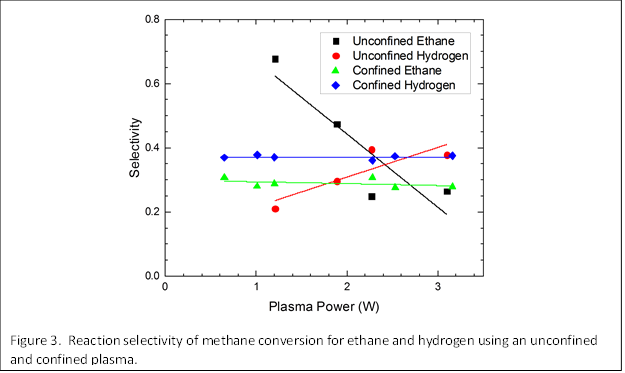
The importance of electrode confinement is shown by a comparative study of CH4 conversion for the unconfined and confined plasma shown in Figure 3. Whereas the selectivity for C2H6 and H2 appears to be strongly power dependent based on results obtained for the unconfined plasma, confining the plasma reveals that the selectivity is independent of power. We suggest that when the plasma is unconfined, changes in power actually change the volume, as observed in Figure 2, which affects the residence time and therefore the selectivity; confining the plasma eliminates changes in volume and the residence time and only looks at the role of power (or power density) which appears to have little effect on selectivity at this process condition.
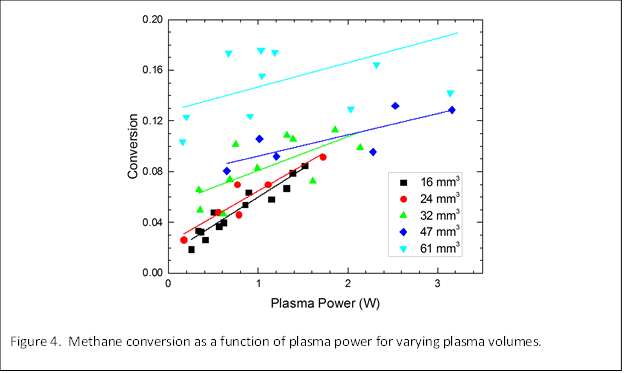
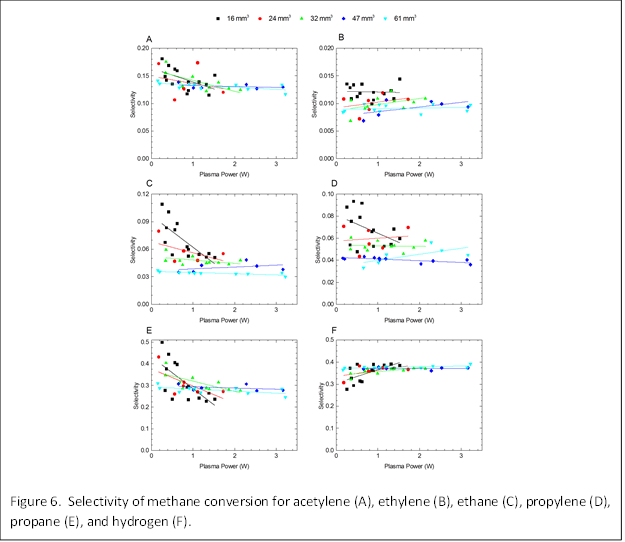
We extended the confined plasma studies to other volumes to further explore the independent roles of volume and power on CH4 conversion and selectivity. Figure 4 shows CH4 conversion as a function of power for several different reactor volumes. We find that conversion increases with power for all volumes, but has a stronger dependence at small volumes, as indicated by the steeper slopes. We note that experimentally, there were limitations in the range of powers that could be tested for the different volumes and only partial overlap of the results could be obtained. Figure 5 shows corresponding selectivities for the various products that were detected by GC analysis. The selectivities for C2 and C3 molecules are found to generally decrease with power while the selectivity for H2 increases with power. These trends were particularly enhanced at smaller volumes (<24 mm3). We suggest that at low powers, the CH4 is not completely dissociated and radicals such as CH3, CH2, and CH are formed that recombined to form ethane, ethylene, and acetylene. At high powers, complete dissociation of CH4 leads to H2 and C.
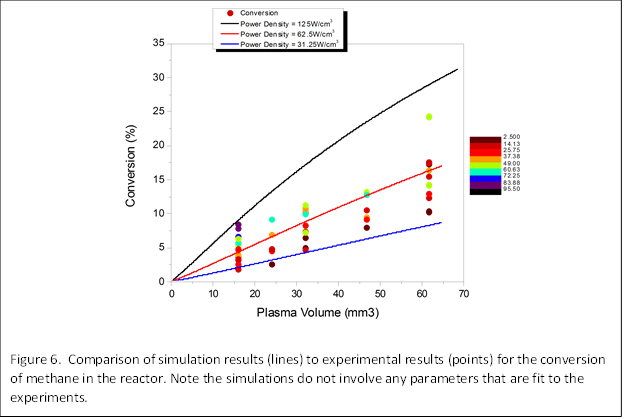
Simulations of the reactor performance were also carried out. These simulations were carried out for the same reactor geometry and conditions used in the experiments. We note that our simulations do not involve any parameters that are adjusted to fit experiment. The simulations proceed by integrating the differential equations for the elementary plasma and chemical reactions that could occur. Approximately 50 reactions are considered. The rate equations for the reactions of the plasma electrons with the molecules are determined by solving the Boltzmann equation. The rate equations for reactions between molecules were obtained from the literature. Results for the conversion as a function of reactor volume are shown in Figure 6. The simulation results (lines shown for three power densities that span the range of power densities used in the experiments) are in good agreement with experiment (points, shown for various power densities).
4. Impact of project
This project has established a new direction of research for the PI, Prof. Sankaran, and Co-PI, Prof. Lacks in the area of gas conversion by plasma-based approaches. Prof. Sankaran has developed experimental techniques for controllably converting gases and characterizing the reactions. Prof. Lacks has developed modeling techniques for predicting the reaction conversion and selectivity. The project is also training a PhD student, Joe Toth. As a result of his progress, this past year Joe was given an invited talk at the International Society on Plasma Chemistry conference in Banff, Canada. A manuscript is also in preparation reporting these results.











