Reports: UNI555164-UNI5: Investigation of Methane Direct Dissociation and Partial Oxidation on Vanadium Surfaces
Heather L. Abbott-Lyon, PhD, Kennesaw State University
Investigation of Methane Direct Dissociation and Partial Oxidation on Vanadium Surfaces
Narrative Progress Report for Year 1
Methane is an abundant and efficient resource within the United States. However, it is dangerous and expensive to transport compared with liquid fuels. Moreover, partially oxidized compounds such as methanol and formaldehyde are important feedstock compounds in the chemical industry. Thus, the conversion of methane to methanol or formaldehyde is highly desirable. Current methods for the conversion of methane to liquid hydrocarbons involve an indirect two-step process. First, syngas (CO + H2) is produced via oxidation of methane. Then, a Fischer-Tropsch reaction is used to create the hydrocarbon of choice. While a direct conversion method would be preferred, it is difficult to perform partial oxidative dehydrogenation rather than complete oxidation to CO2 because of the reaction thermodynamics. Vanadium oxide catalysts are known to be effective for the partial oxidation of methanol to formaldehyde, and it has been shown that this reaction proceeds through a methoxy reactive intermediate. It is hypothesized that a similar methoxy intermediate may be important in the partial oxidation of methane to methanol.
The Abbott-Lyon Laboratory (ALL) is using experimental and theoretical methods to investigate the activity of bare and oxygen pre-covered vanadium surfaces to better understand direct conversion of methane to methanol. In particular, methane dissociative sticking coefficients will be measured as a function of independently varied gas and surface temperatures. In addition, trapped reactive intermediates on the surface will be monitored by reflection-absorption infrared spectroscopy. The non-equilibrium sticking coefficients will be analyzed using a microcanonical unimolecular rate theory or MURT to extract the reaction threshold energy (E0) and other characteristics of the transition state such as the amount of contribution from the surface degrees of freedom (i.e., the number of surface oscillators that participate in the collision complex on the surface).
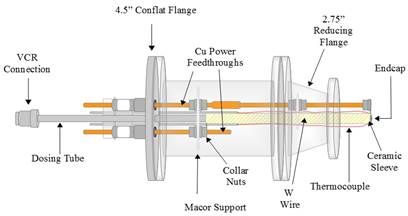
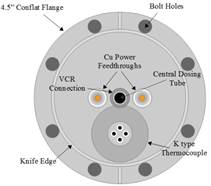
In order to perform the dissociative sticking experiments, a heated effusive molecular beam doser was designed and built during the first year of this award (Figures 1 and 2). The methane gas emitted from the doser will have translation, vibrational and rotational temperatures that are equal to the temperature of the doser nozzle. The doser has recently been installed on an ultrahigh (UHV) vacuum chamber and is being degassed and calibrated at this time. Modifications were made to the vacuum chamber during Year 1 to accommodate the doser and allow dissociative sticking measurements.
Figure 1. Schematic diagrams of the variable temperature effusive molecular beam doser, viewed from the top (left) and from the rear (right), were created in LibreCAD.
Figure 2. Photographs of the variable temperature effusive molecular beam built in the Abbott-Lyon lab. The image on the left shows the bare doser, while the image on the right shows it ready to be attached to the UHV chamber.
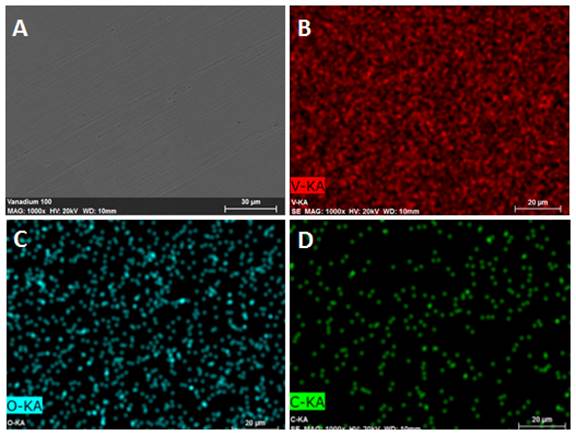
Two vanadium single crystals were purchased from Goodfellows for the planned dissociative chemisorption experiments: V(110), the close-packed surface geometry, and V(100), a more open surface that has been studied in the past. After receiving the polished crystals in February, they were analyzed using scanning electron microscopy with electron backscattering (SEM-EDX) and x-ray photoelectron spectroscopy (XPS) to check the surface morphology and determine the elemental composition in the near surface region (via SEM-EDX) and the surface termination (via XPS).
Figure 3. SEM images of the V(100) sample. The unsputtered single crystal shows a smooth surface morphology (A) with a near surface region consisting of approximately 88% vanadium (B), 7% carbon (C) and 5% oxygen (D).
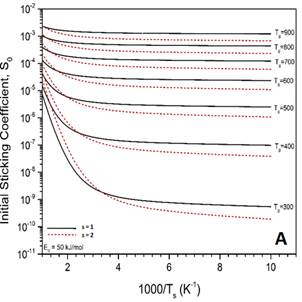
In addition to the experimental work, two undergraduate students ran preliminary MURT calculations with test parameters to ensure that the code is working and to have an estimate of what temperature ranges are most likely to yield observable sticking coefficients. Based on these predictions, sticking coefficient measurements will be performed at a minimum surface temperature of 500 K to give sufficiently high sticking coefficients for optimum results. Sticking coefficients will be measured and analyzed using the MURT during Year 2 of this award.
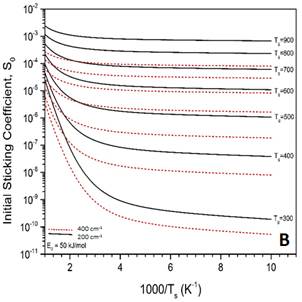
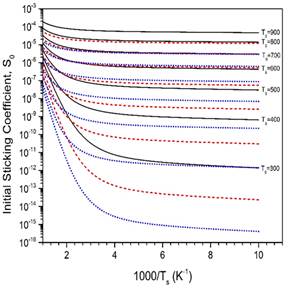
Figure 4. MURT predictions for the dissociative sticking of methane on V(100) assuming the barrier to reaction is 50 kJ/mol. A) Comparison of calculated S0 if the number of surface oscillators is varied while the low frequency vibrations in the transition state are held at 200 cm-1. B) Comparison of calculated S0 if the low frequency vibrations in the transition state are varied while the number of surface oscillators is held constant at s = 2.
Figure 5. MURT predictions of the dissociative sticking coefficient as a function of reaction threshold energy (E0). For these calculations, 2 surface oscillators were assumed and a low frequency of 200 cm-1 was used for the reactive transition state. The barrier heights were varied from 50 kJ/mol (black solid) to 60 kJ/mol (red dashed) to 70 kJ/mol (blue dotted).
To date this award has directly impacted three undergraduate students and one masters student. Undergraduates were involved in all aspects of the project from the design and construction of the heated effusive molecular beam to performing MURT calculations on the methane/vanadium system. They learned to use LibreCAD (a free autoCAD program), to spotweld, to operate and maintain ultrahigh vacuum equipment, and to present their research findings. Two of the undergraduate students who worked on this project were selected to participate in an internally funded, competitive summer research program at Kennesaw State University. One of those students won the poster contest at the end of the summer and was awarded $2000 to present his research at the National Spring ACS meeting in San Diego. The masters student working on this project is expected to defend his thesis and graduate in May 2016. In addition to providing research support for four students, this award also provided summer support to PI Abbott-Lyon so that research rather than teaching could be pursued.