Reports: DNI755378-DNI7: Petroleum Derived Triblock Terpolymer Templates for Dual-Metal Patterning of Square-Patterned Rectangular Nanostructures
Justin G. Kennemur, PhD, Florida State University
SCOPE AND PURPOSE
The ability of block polymer systems to self-assemble into periodic domains tailored by their synthetically chosen size, architecture, and composition presents tremendous promise towards templates for patterning large-area nanostructured arrays. Linear triblock terpolymers, comprised of three unique polymer segments (ABC), attached in a specific sequence, have been shown to afford microphase separated morphologies with alternating cylindrical domains of A and C within a matrix of B.1 Such a morphology provides opportunity to synthetically tailor the A and C segments to selectively absorb metal salts. Successfully producing high-density arrays of multi-metal nanostructures can provide new insights in the frontier areas of lithography, plasmonics, and metamaterials.2 Herein we report our significant process in our first year focused on synthesizing a model triblock terpolymers system and investigations on microphase separation thermodynamics to afford such templates.
RESEARCH PROGRESS
(1) Synthesis of Poly(ethylene oxide-block-styrene-block-2-vinylpyridine)
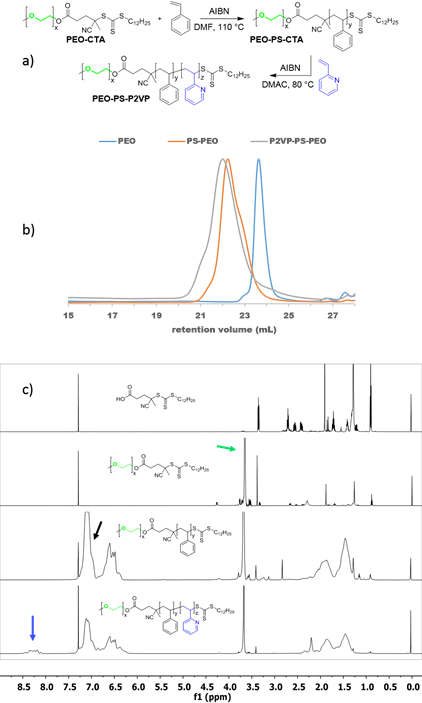
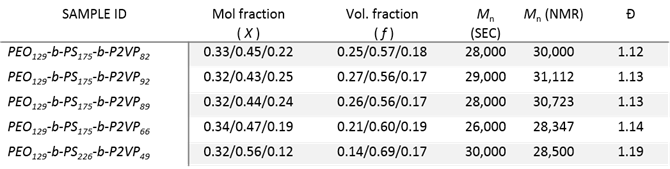
Precedence in literature has shown block polymer systems containing PEO and P2VP segments effectively serve as selective hosts for oppositely charged metal salts.3 To obtain the desired microphase separated morphology of alternating PEO and P2VP cylinders within an inert and mechanically robust third polymer matrix, such as polystyrene (PS), the sequencing of the block segments becomes critical. For this morphology, the PS segment must be the central block flanked by PEO and P2VP on opposite sides. To the best of our knowledge, such a sequencing has never been reported. Figure 1a details the synthetic route our group has undertaken to produce PEO-b-PS-b-P2VP triblock terpolymers using sequential reversible-addition fragmentation-transfer (RAFT) polymerization on a PEO-based chain transfer agent (CTA). Monomethylated PEO was functionalized with a RAFT CTA, 4-cyano-4-(dodecylsulfanylthiocarbonyl)sulfanyl pentanoic acid (CDPA) using N-(3-dimethylaminopropyl)-Nʹ-ethylcarbodiimide (EDC) coupling to produce the PEO-CTA. Subsequent RAFT polymerization of the PEO-CTA with styrene and then 2-vinylpyridine under select conditions then afforded the triblock terpolymers as evidenced by size exclusion chromatography (SEC) and NMR analysis (Figure 1b & 1c). A series of triblock terpolymers were then produced with slightly different volume fractions that were targeted as potential candidates to afford the desired morphology (Table 1).
Figure 1. (a) Synthetic map illustrating the sequential RAFT polymerization of styrene and 2VP starting with a PEO-CTA. (b) SEC chromatograms (THF, 25 °C) showing the progressive increase in molar mass upon addition of each chain segment. (c) Representative stacked 1H-NMR spectra (CDCl3) of (top to bottom) CTA, PEO-CTA, PEO-b-PS-CTA, and final PEO-b-PS-b-P2VP. The green, black, and blue arrows point to unique chemical shifts associated with PEO, PS, and P2VP, respectively.
Table 1. Characterization results of PEO-b-PS-b-P2VP copolymers.
(2) Investigations on microphase separation and morphology
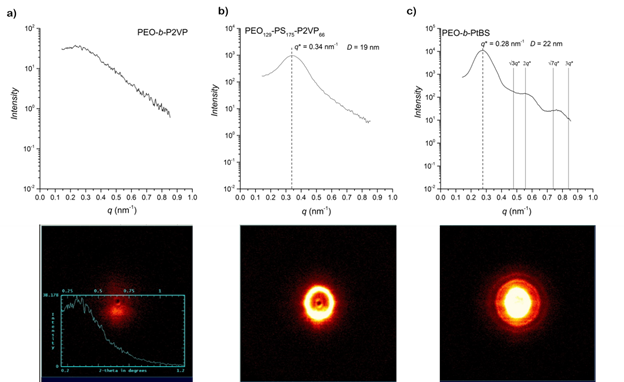
Upon the successful synthesis of triblock terpolymers, our focus has now shifted to understanding their thermodynamics of self-assembly. To achieve microphase separation, the effective segment-segment interaction parameter (χ) between each block (i.e. χA-B, χA-C, χB-C ) must be sufficiently high based on the synthetically chosen degree of polymerization (N) of each segment at a given temperature (T). Furthermore, the volume fraction ( f ) of each segment must be judiciously chosen to afford the desired morphology of alternating PEO/P2VP cylinders. While precedence in literature exists on χPS-PEO4 and χPS-P2VP,5 very little is known about the interaction parameter between PEO and P2VP. As an entry point to begin understanding χPEO-P2VP , we have synthesized a PEO129-b-P2VP118 diblock copolymer (Mn,total = 18 kg mol-1, fPEO = 0.33) through RAFT polymerization using the same PEO129-CTA. Figure 2(a) shows a small angle x-ray scattering (SAXS) profile for the diblock copolymer annealed at 180 °C under vacuum for 24 h and slowly cooled to ambient. The absence of a principle scattering peak suggests well mixed copolymer segments and indicates the overall size of the PEO and P2VP segments in the triblock copolymers will likely need to be increased in order to obtain microphase separation into separate cylindrical domains. Preliminary SAXS analysis on PEO129-PS175-P2VP66 clearly shows a principle scattering peak at q = 0.034 Å-1 (D = 18.4 nm), however, the broadness of this peak and the absence of secondary scattering reflections indicates a disordered state is possible. The other triblock terpolymers in Table 1 exhibited similar SAXS profiles. To ensure secondary scattering peaks can be observed with our in-house x-ray source, a PEO129-b-Poly(tert-butylstyrene) (PtBS)37 diblock (Mn,total = 11.6 kg mol-1, fPEO = 0.46) was also synthesized via RAFT polymerization from PEO129-CTA and the annealed product was analyzed via SAXS (Figure 2(c)). A principle scattering peak of much stronger intensity and secondary scattering peaks found at approximately 2q* and 3q* are consistent with the lamellar morphology expected for symmetrical diblocks.
Figure 2. SAXS 2D-scattering image (bottom) and 1D integration (top) of a) PEO-b-P2VP [Mn = 18 kg mol-1, fPEO = 0.33], b) PEO129-PS175-P2VP and, c) PEO-b-PtBS [Mn =11.6 kg mol-1, fPEO = 0.46].
CURRENT DIRECTIONS & IMPACT
Currently we are synthesizing PEO-b-PS-b-P2VP, PEO-b-PtBS-b-P2VP, and PEO-b-P2VP copolymers with higher segment lengths to access the strong segregation regime for microphase separation. A combination of SAXS and rheology will be performed to determined ordered morphologies and possible order-to-disorder transition temperatures, especially for PEO-b-P2VP where the interaction parameter is unknown and of value to the scientific community. Once the desired materials are realized, thin film casting and annealing studies will begin to align these domains perpendicular to the substrate followed by microscopy analysis (SEM, TEM, AFM) to visualize these ordered structures. Finally, treatment of ordered and aligned thin films with oppositely charged metal salt solutions for selective adsorption into the PEO and P2VP domains will ultimately bring the goals of this project to fruition.
1. Hardy, C. G.; Tang, C. B. J. Polym. Sci. Pol. Phys. 2013, 51, 2-15.
2. Jones, M. R.; Osberg, K. D.; Macfarlane, R. J.; Langille, M. R.; Mirkin, C. A. Chem. Rev. 2011, 111, 3736-3827.
3. Aizawa, M.; Buriak, J. M. J. Am. Chem. Soc. 2006, 128, 5877-5886.
4. Cochran, E. W.; Morse, D. C.; Bates, F. S.Macromolecules 2003, 36, 782-792.
5. Sweat, D. P.; Kim, M.; Larson, S. R.; Choi, J. W.; Choo, Y.; Osuji, C.; Gopalan, P. Macromolecules 2014, 47, 6687-6696.