Reports: UR1055690-UR10: Understanding the Behavior of Guest Molecules within Zeolite Materials
Jennifer S. Holt, PhD, Valparaiso University
Introduction
Host-guest materials can be developed for a variety of practical applications, although a fundamental understanding of the chemistry to improve the desired properties of these materials is often lacking. This project focuses on a particular class of host-guest materials comprised of dye molecules within zeolite hosts in order to better understand the interaction, organization and stability of the guest molecules within the channels of the zeolite crystals. In addition, new nonlinear optical (NLO) properties may develop in these materials if a high concentration of dye molecules are organized and aligned within the zeolite channels. Once these new materials have been fully optimized and better understood, the role of co-adsorbed solvent molecules on dye incorporation into the zeolite channels will also be explored. In addition to generating materials with new properties, this project will increase the fundamental understanding of how molecules behave within zeolite channels in order to improve the use of zeolites within the petroleum industry.
Research Progress
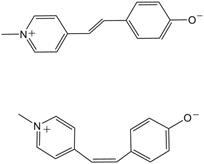
Over the first year of work on this project, we have focused on a single dye-zeolite system in order to understand the factors that lead to maximum dye loading in the zeolite channels. Zeolite L was chosen as the initial host material because of the relatively simple one-dimensional channel structure that has previously been shown to incorporate dye molecules [1]. The dye molecule of interest in this project is Brooker’s merocyanine ((4-[(1-methyl-4(1H)-pyridinylidene)ethylidene]-2,5-cyclohexadien-1-one). Brooker’s merocyanine has zwitterionic character, which may lead to a head-to-tail arrangement of dye molecules within the zeolite channels. It is also known to undergo a unique protolytic/photoisomerization cycle that can be studied spectroscopically in which one step (deprotonated cis form isomerizes to the trans isomer) occurs in only one direction [2]. Therefore, it is an interesting molecule that can be used to probe a variety of conditions within the zeolite channels.
Figure 1: Brooker’s merocyanine in the trans (upper) and cis form (lower)
We successfully and reproducibly synthesized zeolite L, as characterized using x-ray powder diffraction techniques. Once the zeolite was prepared, Brooker’s merocyanine dye molecules were inserted into the zeolite channels by soaking the crystals in an aqueous dye solution. Changes in the x-ray powder diffraction pattern indicated that the dye was incorporated within the zeolite channel and not just adsorbed to the surface of the zeolite crystal grains, although this will also be confirmed with BET porosity studies. The amount of dye adsorbed was quantified using UV/Vis spectroscopy of the dye solution before and after zeolite addition. Once the dye was incorporated into the zeolite, it was difficult to remove.
The first set of experiments to maximize dye loading involved the variation of zeolite L properties that might affect dye incorporation into the channels. Extra-framework cations found within the zeolite channels were exchanged to compare the effect of potassium ions versus smaller hydrogen ions within the channels. No appreciable difference in dye loading was observed between the two zeolite materials. We have also begun work to modify the chemical composition and crystallite size and morphology in order to see if differences in the zeolite structure affects dye loading.
The second set of experiments were performed under a variety of experimental conditions that affected the dye in solution. Optimal conditions of time, temperature and concentration were determined to maximize dye loading from a neutral dye solution. Light exposure and pH affects the isomerization of the dye molecule, so these variables were also studied. Acidic dye solutions were used to quantify the incorporation of the protonated dye molecules in the cis (exposed to UV light) and trans (kept in the dark) forms. Initial results indicated that more dye was adsorbed from the solution containing the protonated cis isomer than from the solution containing only the protonated trans isomer. This will be verified in the near future. The relative rate of incorporation of both isomers will also be studied, as well as the stability of the cis form within the constrained environment. The maximum dye loading achieved to date is approximately 8x1019 dye molecules per gram of zeolite. This dye loading is sufficient to lead to NLO properties if the molecules are aligned within the channels, so these materials will be tested for NLO response.
Impact on PI and Students
Since beginning the dye-zeolite project at Valparaiso University, four undergraduate students have been involved. Three undergraduate research students were supported through the grant during the summer of 2016. As sophomores, they have gained valuable experience in a variety of instrumental techniques and synthetic methods not learned in the classroom, and they have formed a collaborative research group that will continue to grow as this project moves forward. Two of these students have indicated that their research experience has enhanced their interest in a research career, while the other has become interested in pursuing a career at the intersection of science and business. Each of these students have presented their results on campus, and their work was included in three regional and national presentations. Travel money was used to send another student who performed initial experiments during the academic year to the Indiana Academy of Science meeting. The presentation of this work at that meeting led to a potential collaboration within the state to study our dye-zeolite materials using his nonlinear optical set-up, which will allow my students to be present when we determine if their samples are NLO-active. This collaboration will be a great asset to our research beyond the time of this grant.
[1] Calzaferri, G., Nanochannels: Hosts for the supramolecular organization of molecules and complexes. Langmuir, 2012, 28, 6216-6231.
[2] Steiner, U., Abdel-Kader, M.H., Fischer, P., and Kramer, H.E.A., Photochemical cis/trans isomerization of a stilbazolium betaine. A protolytic/photochemical reaction cycle. Journal of the American Chemical Society, 1978, 100, 3190-3197.