Reports: ND653752-ND6: Solid-State NMR Studies of Heterometallic and Homometallic Organic Frameworks
David L. Bryce, PhD, University of Ottawa
The second and final year of our ACS PRF New Directions project yielded several important breakthroughs in the areas of solid-state nuclear magnetic resonance (NMR) spectroscopy, particularly as applied to metal nuclei, to metal-metal bonds, and to metal-organic frameworks and related coordination polymers. Several students and two post-doctoral fellows have contributed to this work during the reporting period. Three new papers related to the project have been published so far since the last report. Further manuscripts are currently being finalized for submission for publication.
Our project aims to develop and apply advanced multinuclear magnetic resonance methods to study and characterize metal-organic frameworks (MOFs) and related functional materials. In addition to developing and applying methods to characterize such systems, another goal of the PRF work was to move into the area of paramagnetic samples. Paramagnetism can cause problems with NMR spectra in part due to the strong dipolar coupling between nuclear and electronic spins and/or fast relaxation induced by the unpaired electrons. This past year, we have published our first work in the area of paramagnetism, with more results in the pipeline. In a collaboration which also involved various other methods including electron paramagnetic resonance and Mossbauer spectroscopies, a unique pentacoordinate architecture around Fe(III) ions in interpenetrated metal-organic framework MOF5 was probed via solid-state NMR. Carbon-13 and very fast magic-angle-spinning proton solid-state NMR was used to confirm the presence of the known diamagnetic MOF5 structure as well as paramagnetic Fe(III) ion linkers intercalated within the structure (Figure 1). Further work on other paramagnetic systems is in progress. Our solid-state NMR methods have offered unique insights into these important materials.
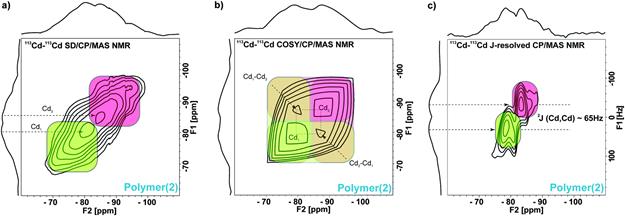
A second application to the study of framework materials and related coordination polymers has revealed how one can map and understand the coordination chemistry of cadmium(II) in a series of systems. In particular, cadmium-113 chemical shifts were employed to understand the changes in structure and coordination for a series of solids existing as molecules, polymers, or metal-organic frameworks. Of particular interest was the observation of an exceptionally rare cadmium-cadmium coupling of about 65 Hz from a series of two-dimensional correlation NMR experiments. A spin diffusion experiment as well as an independent 113Cd-113Cd COSY experiment both revealed proximities between two cadmium nuclei in the one-dimensional coordination polymer [{Cd2(TpymT)(NO3)4}n]. A further two-dimensional J-resolved experiment provided the value of the coupling constant (Figure 2). These experiments and results are valuable for probing such functional materials, particularly in the case of the polymer, where disorder may preclude the application of standard diffraction-based techniques.
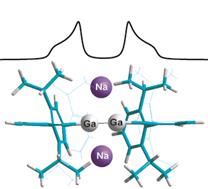
The final published work from the reporting period represents a true breakthrough in the understanding of metal-metal bonding and also pushes the limits of what types of information may be gleaned from the solid-state NMR spectroscopy of quadrupolar nuclei. Building on our groundwork from 2015, we developed and implemented a series of two-dimensional J-resolved experiments for quadrupolar nuclei which work on stationary powders. This is particularly challenging given the very large (e.g,. several MHz) spectral linewidths for many nuclides. Approximately 70% of all nuclides in the periodic table are quadrupolar, and so the methods we have reported should be particularly useful to address a wide range of problems in the future. Presently, we focussed on addressing the long-standing issue of multiple bonding between heavier elements, in particular multiple bonds between gallium atoms (Figure 3). Last year, we showed how multiple bonds between boron atoms behave analogously to multiple carbon-carbon bonds. But what about the heavier gallium congeners? Our experiments, which required the National Ultrahigh-Field NMR Facility for Solids in Ottawa to succeed, were carried out in a magnetic field of 21.1 T. The results clearly showed that, according to the spin-spin couplings measured between gallium atoms in a purported series of gallane, gallene, and gallyne compounds, the bonding paradigm differs when compared to well-defined boron-boron or carbon-carbon multiple bonds. This work was published as a ‘hot paper’, and contributes both to advances in NMR spectroscopy of metal-containing systems, but also to our fundamental understanding of chemical bonding in solids.
The impact of this project on the careers of the principal investigator and of the students and postdoctoral fellows involved has been substantial. For example, in the past year I have been appointed as the University Research Chair in Nuclear Magnetic Resonance. Former student F. Perras was awarded three prestigious prizes in part for the work he has carried out on this project: the Governor General’s Gold Medal in Science and Engineering, the 2015 IUPAC/Solvay thesis prize, and the 2016 Raymond Andrew Prize of the Ampere Society for excellence in magnetic resonance. Former postdoctoral fellow L. Kobera has secured a full-time research position at the Institute of Macromolecular Chemistry at the Academy of Sciences of the Czech Republic. Postdoctoral fellow Jamie Frost is now a research associate at the University of Glasgow.
Figure 1. Experimental 13C very fast magic-angle-spinning (MAS) NMR (a) and 13C cross-polarization MAS NMR (b) spectra of MOF5 with intercalated Fe(II) centres (2.5 % iron content). New signals are observed in (a) which are attributed to the effects of paramagnetism of the iron centres. From Chem. Eur. J., 22, 7711-7715 (2016).
Figure 2. Two-dimensional 113Cd–113Cd homonuclear correlation experiments for a cadmium-containing coordination polymer acquired at B0 = 11.7 T. (a) 113Cd–113Cd spin diffusion CP/MAS NMR experiment with a spin diffusion time of 50 ms. (b) Symmetrized 113Cd–113Cd COSY CP/MAS NMR experiment. (c)113Cd–113Cd J-resolved CP/MAS NMR experiment. From Chem. Commun., 2016, 52, 10680-10683.
Figure 3. Multiple bonding between pairs of gallium atoms in solid compounds was probed using a two-dimensional NMR experiment at high magnetic field. From Chem. Eur. J., 22, 9565-9573 (2016).