Reports: UR1053827-UR10: Bringing Undergraduates Into Lab-Based Research and Development (BUILD) for Effective Shale Gas Storage Using Macroporous Adsorption Complexes (MACs)
Jingbo Liu, PhD, Texas A&M University-Kingsville
Introduction: The shale potential as a source of natural gas has been studied in many countries. The US and Canada have significant shale gas production although other countries have large amount of the recoverable shale resources. It was projected that the natural gas from the shale will grow from 42 BCF/D in 2015 to 168 BCF/D in 2040 and shale is anticipated to account for 30% of world natural gas production. One of the major components composed of the shale gas is methane (CH4). Therefore, it is critical to develop materials to improve CH4 storage. The main features of CH4 (at room temperature, CH4 supercritical, Tc = 190.6 K, Pc = 45.8 atm) indicated that CH4 cannot be liquefied by compression above Tc. It is challenging to store the large amount of CH4 in a given volume for its application (such as gas-driven automobile). Preparation of porous materials therefore becomes important to achieve its end application. The ideal materials need to display the following properties: 1) high surface area, 2) large pore volume, 3) low density, and 4) a strong interaction with the adsorbent.
Completed activities: This report presented series materials, metal-organic frameworks (MOFs) and caged porous composites (CPCs) prepared by a feasible wet-chemistry. The synthesis variables were optimized to control the porosity, size and shape of nanocomposites. Those materials were characterized using the state-of-the-art techniques to evaluate their nanostructure, porosity and gas uptake. The materials safety was also evaluated by measurement of toxicology with a focus on the cellular assays.
Materials preparation: The colloidal method was used to produce Zn-MOFs, Co-MOFs, Ag-MOFs, Fe3O4-CPCs and ZnO-CPCs. Due to the attractive van der Waals interaction, the colloid tends to aggregate, resulting in particles growth. To prevent particle growth, the amphiphilic dispersing agents were introduced to balance the attractive and repulsive forces. The long-chain molecules (e.g. starch, Gum Arabic) were introduced to achieve steric stabilization via chemical bond or physical attraction, creating a repulsive force due to the steric hindrance.
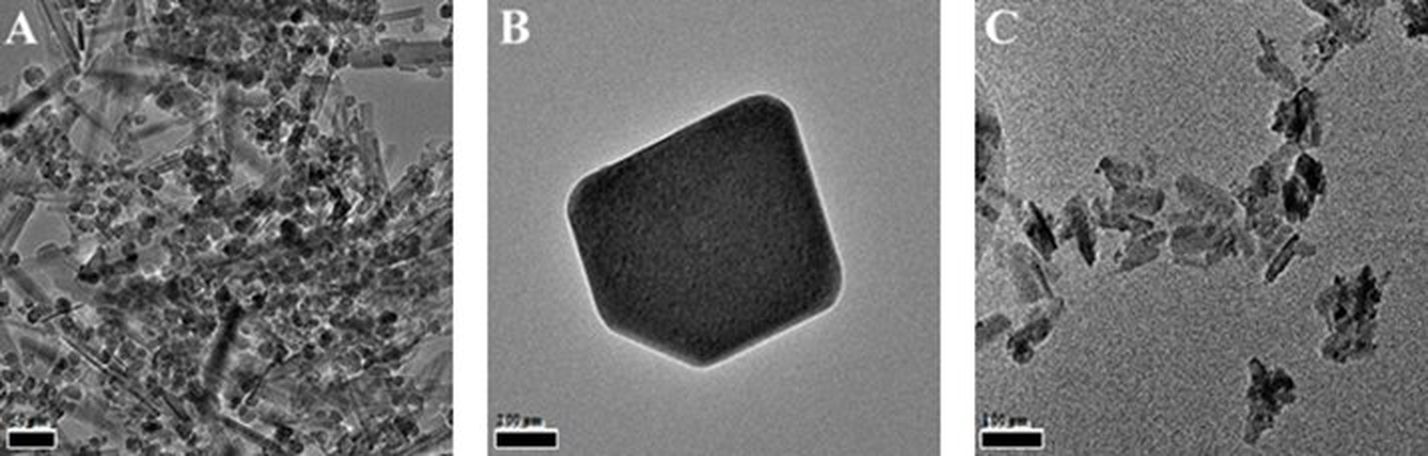
Materials characterization: The morphology and fine structure of the MOFs and CPCs were characterized using a transmission electron microscopy (TEM, FEI Company, Tecnai F20-G2, Hillsboro, OR). The TEM images of three series nanocomposites (Fig. 1A-C) indicate that the shapes of the CPCs (totally 89 formulations) were controlled as rod-shaped with an average diameter at 10 nm and length at 78 nm (Gum Arabic shielded Fe3O4, Fig. 1A), cubic (Aloe Vera modified ZnO, Fig. 1B), and fibrous (Starch modified ZnO, Fig. 1C) depending on the shielding agents and heat-treatment conditions. The Ag-MOFs are highly crystalline, however, the Cu, Co and Zn centered MOFs did not show desired structure nor the gas uptakes. The amount of MOFs also needs to be scaled up for gas storage (a focus in next report).
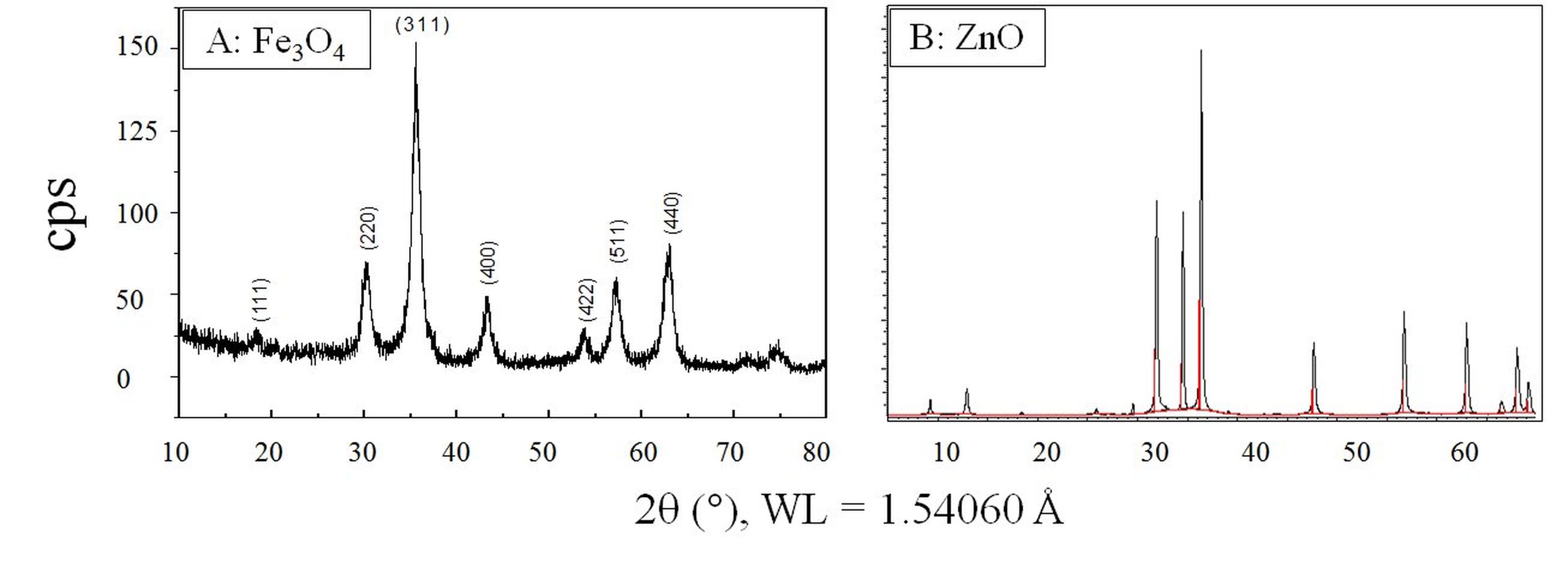
The powder X-ray diffraction (PXRD, Bruker-AXS D8 Vario X-ray Powder Diffractometer, Madison, WI) indicated that the Fe3O4-CPCs (Fig. 2A) displayed an inverse spinel structure (PDF 65-3107, a = 8.3905Å, α = 90 °). The ZnO-CPCs (Fig. 2B) were tetragonal and well-indexed with the standard PDF 89-0511 and PDF 05-0664 (a = 3.249, c = 5.205, α = 90 °). Briefly, the nanocomposites were generated by a colloidal chemistry to produce well-defined and highly crystallized compounds. The crystal unit nucleation was found to be promoted when the solvents were evaporated gradually. This controllable nucleation favored the tunable size and monodispersity. It was also found the natural product extracts play a critical role in the particles shape and porosity.
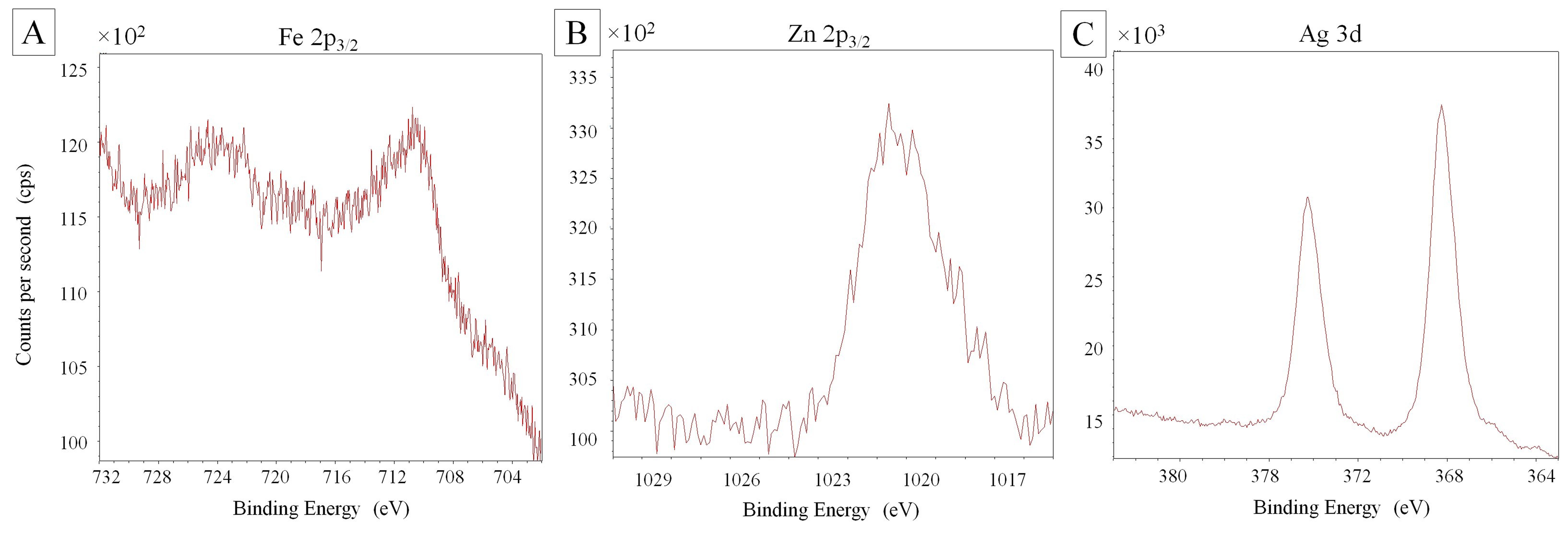
The X-ray photoelectron spectroscopy (XPS, Omicron ESCA+, Scienta Omicron, Inc., Denver, CO) was used to determine the elemental composition. The chemical state of Ag, Fe, Zn, C and O atoms can be determined from the binding energy (BE) and chemical shifts. The XPS spectra of the nanocomposite (Fig. 3A-C) indicated the presence of the Fe, Zn and Ag; and O and C elements and their compositions.
Gas uptake: The BET absorption model and techniques (Micromeritics ASAP 2020, Norcross, GA) were applied to measure the gas-uptake. CH4, CO2, H2 and N2 were used to evaluate the gas selective adsorption. The measurement temperatures are controlled at 87, 175, and 231 K, respectively. Before adsorption measurement, the samples were activated using the “outgas” function of the adsorption analyzer for 12 hours at 80 °C. The surface area (averaged at 536.8 m2/g) allows for gases can effectively penetrate. However, the porosity (67.6) was found to be decreased due to the introduction of functional groups (such as alpha acetal from the starch coil structure).
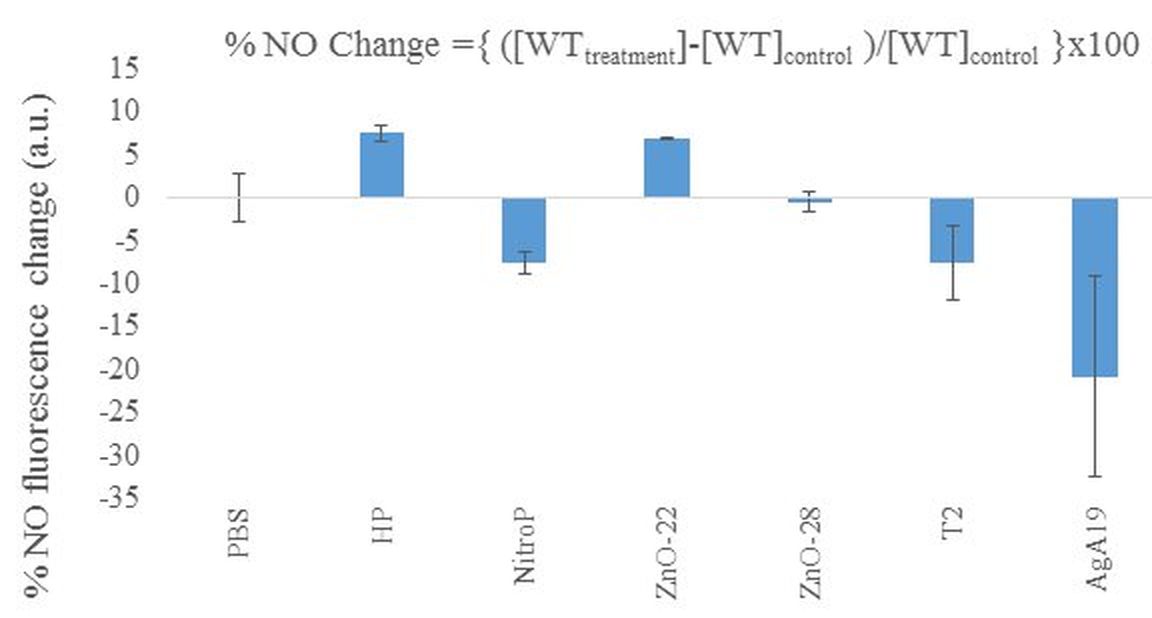
Human Health Impact: The comprehensive review indicated that the natural gas causes 1-11 death per TWh. In the USA, the casualty was found to be 10-20 from oil and gas extraction. Therefore, it is important to evaluate the safety of materials used in the shale gas storage. The retinal pigment epithelium wild type (WT) cells were analyzed with the following standards: 1) control group, 2) an oxidizing (HP) and 3) nitric oxide (NO) releasing agent (NitroP). The materials used for CH4 storage, CPCs (ZnO-22 (10 mg/mL), ZnO-28, Fe3O4-T2), and MOFs (Ag-A19) were evaluated for comparison with the above standards (Fig. 4).
It was found the addition of HP resulted in a relative increase in NO. The addition of NitroP releases NO and results in the activation of the negative feedback loop, lowering NO levels, under biological conditions of high NO. The nanocomposite Zn-28 has near-zero effect in terms of NO generation and appears to be non-toxic. The use of ZnO-22 appears to be slightly oxidizing, but insufficient to cause cellular damage. The increase in NO displays a protecting effect against further oxidative stress. The changes of NO after addition of Fe3O4-T2 suggests a similar mechanism to NitroP. Both chemicals are able to translocate to the mitochondria and directly affect the redox states of the Fe and Cu centers in respiratory enzymes. The Ag-A19 MOF was found to be highly destabilizing and caused cross-linking in the mitochondria membrane, due to the silver center, resulting in a drop of NO.