Reports: UNI155237-UNI1: Supramolecular Catalyst Assembled through Aromatic Donor-Acceptor Interaction and Its Application in Hydrolytic Kinetic Resolution of Epoxides
Yu Liu, PhD, Northern Michigan University
Supramolecular catalysts refer to multicomponent assemblies that are stabilized by noncovalent interactions to achieve catalytic systems with high selectivity and high catalytic efficiency. The common noncovalent interactions used in the assembling supramolecular catalysts include metal-ligand complexation, hydrogen bonding, cation-anion attraction and ion-dipole interaction. The aromatic donor-acceptor interaction not only has a higher binding affinity and more precise control of spatial arrangement than the regular π- π interaction, but also tolerates a wide range of solvent from cyclohexane to water. It has been applied extensively in the constructions of a variety of supramolecular assembles. In this project, we explored the assembly of supramolecular catalysts based on the aromatic donor-acceptor interaction, which has not been reported.
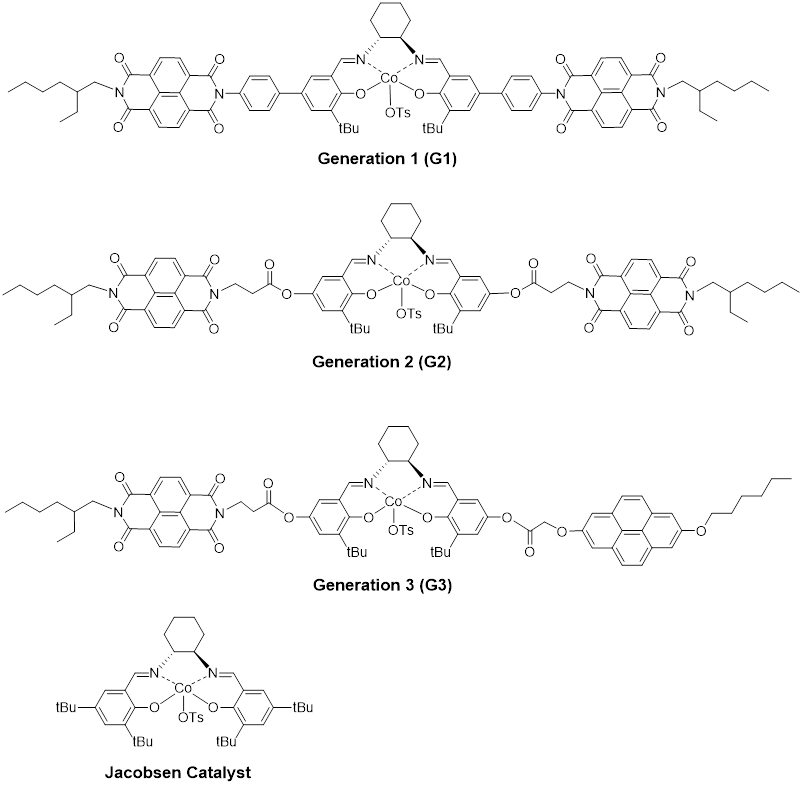
Figure 1. Three generations of synthesized Co(III)-salen complexes and the commercial Jacobsen Catalyst.
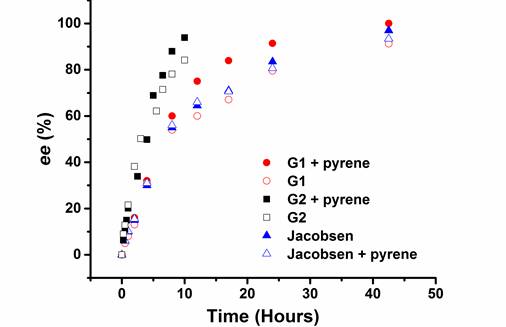
We designed and synthesized Co(III)-salen complexes containing aromatic acceptor units (or acceptor & donor units). The aromatic donor-acceptor interaction drives the formation of a di-nuclear Co(III)-salen supramolecular catalyst, which can catalyze the bimetallic mechanism based hydrolytic kinetic resolution (HKR) of epoxides. We began the project with the synthesis of Co(III)-salen complex G1 (Generation 1, Figure 1), in which a pair of electron-deficient 1,4,5,8-naphthlenediimide (NDI) (acceptor) are attached to the central Co(III)-salen core via a rigid phenyl linker. G1 was tested in HKR of epichlorohydrin under a variety of reaction conditions, such as different solvents and different donor compounds (pyrene, 1,5-dimethoxynaphthlene, perylene and coronene). Under the optimized condition, we observed an increasing reaction rate by introducing pyrene in the reaction system. With 3.5 equivalence of pyrene to G1, the reaction was complete in 42.5 hours with > 99% ee, in contrast to 91 % ee of the reaction without pyrene. This result implied that the aromatic donor-acceptor interaction between pyrene and Co complex facilitated the assembly of the di-nuclear supramolecular catalyst. However, when compared with Jacobsen catalyst (97 % ee in 42.5 hours), the catalysis efficiency of G1 did not show much advantage. We further designed and synthesized the second generation of Co(III)-salen complex (G2, Figure 1). It is featured with a flexible and non-conjugated alkyl ester link. Under the same reaction condition, G2 demonstrated a remarkable rate enhancement. When 3.5 equivalence of pyrene was applied, the HKR of epichlorohydrin was complete in 12 hours. When no donor compound was introduced, the reaction took more than 14 hours to complete, which, again indicated the donor-acceptor interaction enhanced the dinuclear supramolecular catalyst assembly. The following kinetic plot illustrates the catalytic activity comparison of G1, G2 and Jacobsen Catalyst (Figure 2).
Figure 2. Kinetic plot of the HKR of epichlorohydrin by Co(III)–salen catalysts (pyrene is in 3.5 equivalence to salen complexes if used. CHCl3 is used as the solvent).
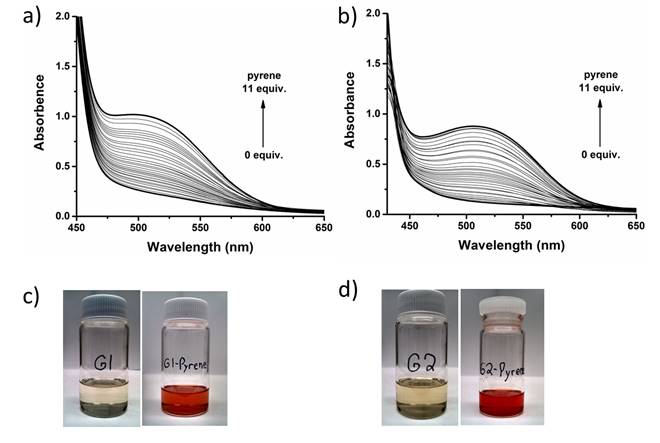
To further verify the proposed aromatic donor-acceptor interaction based supramolecular catalyst assembly, we did a series of UV-vis absorption and NMR spectroscopy titration studies. The aromatic donor-acceptor interaction is featured with a charge-transfer band in UV-vis spectra. Figure 3a and 3b shows the evolution of broad absorption bands with a maximum at 505 nm for G1 and 510 nm for G2, upon the addition of pyrene, characteristic of the charge-transfer transition. The appearance of a long wavelength charge-transfer absorption band renders solutions of G1+pyrene and G2+pyrene red in color (Figure 3c and 3d). The face-to face stacking of donor and acceptor also results in upfield shifts of the aromatic protons because of the shielding effect in 1H-NMR spectra. In both G1 and G2 1H-NMR titration experiments, upfield shifts of NDI protons and pyrene protons were detected. These titration experiment results provided the additional evidence of the aromatic donor-acceptor interaction involved supramolecular catalyst assemblies. Currently, we attempt to extract the association constants and other information from UV-vis spectra and NMR spectra.
Figure 3. (a) The UV-vis spectroscopy titration curves of G1 with pyrene (the starting curve and the end curve are highlighted in bold), (b) The UV-vis spectroscopy titration curves of G2 with pyrene (the starting curve and the end curve are highlighted in bold), (c) The photographs of G1 solution and the titration ending G1-pyrene solution, (d) The photographs of G2 solution and the titration ending G2-pyrene solution.
Based on the experience from G1 and G2 catalysts, we further optimized the design and synthesized the third generation of Co(III)-salen complex (G3, Figure 1). Different from G1 and G2, G3 contains both the donor unit (pyrene) and the acceptor unit (NDI) in a same complex, so the self-assembly of the binuclear supramolecular catalyst can be realized by G3. The initial HKR test was very encouraging. Under the same reaction condition as G1 and G2, G3 significantly promoted the reaction rate and the HKR of epichlorohydrin was done in 3.5 hours. We are now carrying more detail kinetic studies and also testing more epoxides for G3.
During this funding period, six NMU undergraduate students worked on this project. Three of them got the stipends funded by this PRF: Aaron Yoder, Daniel Blechschmidt and Daniel Lilly. Two posters on this work have been presented (2016 ACS Upper Peninsular Local Meeting in Marquette, MI and 2016 ACS Central Regional Meeting in Covington, KY). A manuscript is also under preparation.