Reports: UR153693-UR1: Intramolecular Cycloaddition Reactions of Electron-Rich Alkynes: New Methods for the Synthesis of Optically Active Building Blocks for Organic Synthesis
Thomas G. Minehan, California State University (Northridge)
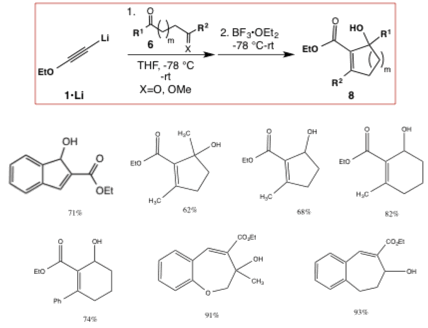
Our investigation of the chemistry of electron-rich alkynes (including ynamines and ynol ethers) has led to the discovery of two new fundamentally important transformations. First, ynol ethers can be combined sequentially with epoxides or oxetanes and carbonyl compounds in a one-pot process in the presence of a Lewis acid to form five- and six-membered Z-alpha-alkylidene and Z-alpha-benzylidene–gamma-butyrolactones and -delta-valerolactones in good to excellent yields. The alpha-alkylidene lactone moiety is found in numerous synthetically challenging and biologically important natural products, many of which possess anticancer, antimalarial, antibacterial, antifungal, antiviral, and/or anti-inflammatory activities. This single-flask reaction achieves the formation of three new carbon-carbon bonds and a ring, and likely occurs via alkyne-carbonyl metathesis of alpha-hydroxy-ynol ether intermediate, acid-promoted alkene E- to Z- isomerization, and lactonization (Scheme 1).
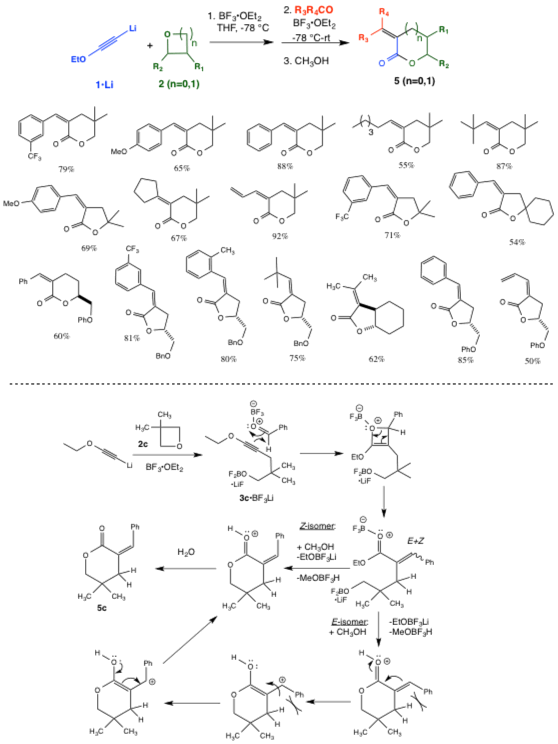 |
Scheme 1. One-Pot Synthesis of a-alkylidene and -benzylidene-g-butyrolactones and -d-valerolactones.
As an extension of this method, we have found that ynol ethers can also be combined sequentially with two different carbonyl compounds in the presence of BF3-OEt2 to furnish diverse Morita-Baylis Hillman adducts. Mechanistically, the reaction proceeds through reversible formation oxonium ions B and C (Scheme 2), furnishing the most stable alkene product in excess.
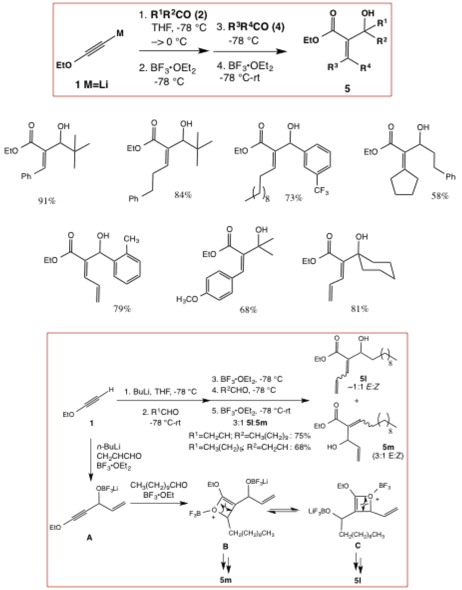
Scheme 2. Synthesis
of Morita-Baylis Hillman adducts
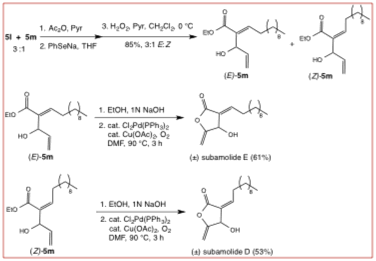
Adducts E-5m and Z-5m can be transformed into the natural products Subamolides D and E (Scheme 3), two naturally occurring alpha-alkylidene lactones which have been shown to be DNA damaging agents capable of inducing apoptosis in malignant cells, by a short reaction sequence involving ester hydrolysis and palladium-catalyzed oxidative cyclization.
Scheme 3. Synthesis of Subamolides D and E.
Cyclic Baylis Hillman adducts can also be efficiently prepared by the reaction of ethoxyacetylene with dialdehyde, diketones, and aldehyde/keto acetals in the presence of Lewis acid (Scheme 4).
Scheme 4. Synthesis of cyclic Morita-Baylis-Hillman adducts.
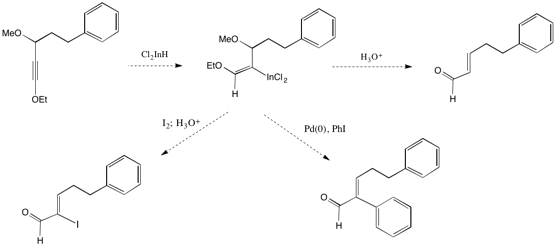
Currently we are exploring the regioselective addition of dichloroindium hydride to ynol ethers. The intermediate metalated enol ether may undergo reactions with electrophiles or palladium-catalyzed cross-coupling reactions to provide alpha-functionalized enals in high yields (Scheme 5).
Scheme 5. Exploring the regioselective addition of dichloroindium hydride to ynol ethers.
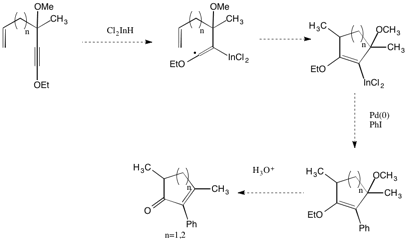
Since the addition of indium hydride to alkynes is a radical process, we are also exploring trapping of the intermediate stabilized radical with pendant alkenes in a 5-exo-trig or 6-exo-trig cyclization process. Subsequent reaction of the organoindium species with electrophiles is expected to provide complex cycloalkenones in a single-flask reaction (Scheme 6).
Scheme 6. Indium-promoted tandem radical cyclization/cross-coupling reactions of ynol ethers.
Finally, we are investigating the utility of ynol ethers for the facile synthesis of exo-glycals from carbohydrate lactones (Scheme 7). Exo-glycals may be useful in the synthesis of diverse C-glycosides.
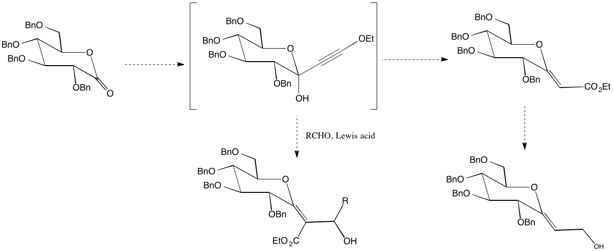
Scheme 7. Synthesis
of exo-glycals
from ynol ethers.