Reports: UR554214-UR5: Mechanistic Studies of Polyelectrolytes for Efficient Scale Inhibition
Xin Wen, PhD, California State University, Los Angeles
The formation of sparingly soluble and insoluble inorganic salts (i.e., scale deposits) is a major problem in the petroleum industry operations. To avoid formation damage and well productivity reduction, it is crucial to remove scale deposits and control scale growth. The use of chemical scale inhibitors (i.e., antiscalants) is a common method for controlling scale deposits.
Commercial scale inhibitors for divalent cation carbonate and sulfate scales, such as calcium carbonate [(CaCO3) calcite and aragonite] and barium sulfate [(BaSO4) barite], include the polyelectrolytes that can dissociate phosphonates, carboxylates, and sulfonates anionic groups. However, it is imperative to identify highly efficient polymeric inhibitors and environmentally friendly ones to replace phosphonate inhibitors due to their environmental risks.
The efficiency of a polyelectrolyte inhibitor mainly depends on the properties of the charges, such as the strength, the number, the availability, and the conformation of the charges. Certain polypeptides with negatively and/or positively charged groups have been extracted from organisms and found to efficiently control the nucleation and crystallization of minerals (e.g., calcium carbonate). Their structures are attractive models for better understanding the inhibitor-mineral interactions and designing next generation antiscalants.
Antifreeze polypeptides (AFPs) from cold-adapted organisms (e.g., fish, insects, and plants) are known to inhibit the nucleation and crystallization of ice as well as certain non-ice like compounds. In fact, analogs of the alpha-helical type I AFP from winder flounder have been found to control the crystal growth of calcite.
In this project, we aim to correlate the charge and molecular properties of the polyelectrolytes with their efficiencies in inhibiting the scale crystal formation.
In Year 2, we further examined the effects of charged residues on the surface of a natural polypeptide on calcium carbonate crystallization. An AFP from the Tenebrio molitor beetle (Mealworm) (TmAFP) is investigated.
Considered as an analog of DAFP, TmAFP has over 60% sequence identity and very similar structure with DAFP. One molecule of TmAFP has the following charged residues: 4 aspartic acids, 1 glutamic acid, and 3 lysines, the side chains of which are on the surface of the polypeptide.
Our studies on DAFP suggest that the side chains of three out of the five aspartic acids form potential salt bridges with the side chains of arginines. These interactions may thus alleviate the effect of DAFP on calcium carbonate crystallization. Comparing with DAFP, TmAFP has comparable number of negatively charged residues, but no arginines. We expect that the charge residues (e.g., aspartic acids) in TmAFP have more potent effects on calcium carbonate crystallization.
Similarly, we prepared TmAFP and investigated the characteristics of calcium carbonate (CaCO3) formation by mixing of equal volumes of equimolar sodium carbonate (Na2CO3) and calcium chloride (CaCl2) in the absence and the presence of TmAFP at room temperature.
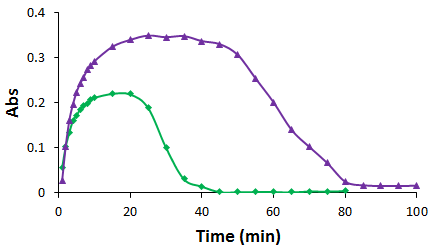
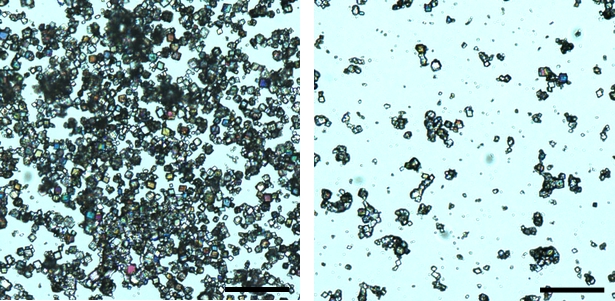
The formation of CaCO3 particles and the suspensions of the formed CaCO3 particles were monitored by absorbance. And as expected, the effects of TmAFP on CaCO3 crystallization are more significantly than those of DAFP. The presence of TmAFP results in a dramatic right shift of the maximal absorbance (Figure 1), suggesting that the precipitation rate of CaCO3 greatly decreases by TmAFP. The presence of TmAFP also significantly increases of the sedimentation time (Figure 1), indicating that the precipitated CaCO3 crystals in the presence of TmAFP are with smaller sizes. The results suggest that favorable interactions exist between TmAFP and CaCO3 crystals. The micrographs of the CaCO3 crystals achieved in the absence (as a control) and presence of the electrolyte at a 10-5 molar ratio are shown in Figure 2. Much fewer CaCO3 crystals are formed in the presence of the electrolyte.
Figure 1. A typical graph of the light absorbance changes of the systems in the absence (green diamonds) and the presence (purple filled triangles) of the prepared electrolyte, TmAFP, versus time.
Figure 2. Optical micrographs of achieved CaCO3 crystals in the absence (left) and presence (right) of the prepared electrolyte, TmAFP. The scale bar is 100 µm.
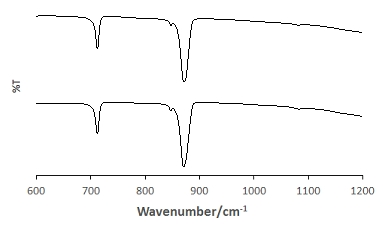
There are three common crystalline forms of CaCO3, namely, vaterite, aragonite, and calcite, in the order of decreasing solubility. These polymorphs of CaCO3 can be distinguished by using infrared (IR) spectroscopy. Here, Fourier transform infrared (FTIR) is used to characterize the form of the achieved CaCO3 crystals and the FTIR spectra are shown in Figure 3. The characteristic calcite bands (i.e., 874 cm-1 and 713 cm-1) are shown in the CaCO3 control and in the presence of TmAFP (Figure 3), suggesting that the presence of the electrolyte does not change the form of CaCO3 crystals.
The potential interactions between the surface residues of the electrolyte, TmAFP, and the crystalline surfaces of calcite is under study.
Figure 3. FT-IR spectra of achieved calcium carbonate crystals in the absence (top) and presence (bottom) of the electrolyte, TmAFP. The bands observed at 874 cm-1 and 713 cm-1 are characteristic carbonate ν2 and ν4 bands suggesting that the presence of the electrolyte does not change the form of CaCO3 crystals.
This support has not only enabled new discoveries in the field of scale inhibition by the PI and her students, but also exposed more students who have interests in science to experience chemical research. Such research experience has inspired these students to pursue STEM careers. Moreover, it is noteworthy that most of these students are underrepresented minorities.
Two undergraduates, Audrey Kishishita and Joshua Lugo, presented their research entitled, “Effects of antifreeze polypeptides on calcium carbonate crystallization”, at the 251st American Chemical Society Meeting in March 2016, San Diego, CA. Moreover, their poster presentation was selected to be presented at Sci-Mix at the National American Society in spring 2016. They were the few undergraduate students presented research at Sci-Mix. We are preparing manuscripts on this project.
The activities supported by this grant have great positive impacts on the students’ lives as well as on the career of the PI as a researcher, teacher, and mentor.