Reports: DNI555519-DNI5: Nanostructured Catalysts for Tandem Catalysis Prepared by Atomic Layer Deposition
Xinhua Liang, PhD, Missouri University of Science and Technology
Heterogeneous catalysts have been used to manufacture most chemicals (e.g., making gasoline from petroleum, producing fertilizers). However, heterogeneous catalysts frequently lack the ability to convert specific molecules in a mixture of reactants and cannot selectively catalyze desired reactions, such as favoring olefin hydrogenation at high conversion. Highly efficient heterogeneous catalysts are required for these reactions. Atomic layer deposition (ALD), a layer-by-layer process, has received increasing attention for preparing metal nanoparticles, such as Pt and Pd. Due to their high cohesive energy, highly dispersed metal nanoparticles tend to form via an island growth mechanism (i.e., Volmer-Weber mechanism) during the initial stages of ALD processes. In the past year, this ACS PRF DNI-supported research has focused on the synthesis of metal nanoparticle catalysts by ALD for gas phase reactions. Two catalysts were prepared and studied to assess their catalytic performance, one was CeO2/Pt/SiO2 for tandem reactions of methanol decomposition and ethylene hydroformylation to produce propanal, and the other was nickel nanoparticles for dry reforming of methane (DRM).
It is estimated that the United States has about 42 trillion cubic meters of shale gas (mainly CH4) trapped in shale rock. Rising energy consumption and depleting reserves have spawned a concerted drive to promote efficient usage of CH4. It is advantageous to convert CH4 into small olefins (e.g., ethylene or propylene) and then convert these olefins into carbonyls and alcohols via hydroformylation reaction. Normally, multi-steps are needed for such conversion. In this research, we are trying to prepare a bilayer of CeO2/Pt/SiO2 nanoparticles by ALD. The two distinct metal-metal oxide interfaces, CeO2-Pt and Pt-SiO2, can be used to catalyze two distinct sequential reactions. The CeO2-Pt interface can catalyze methanol decomposition to produce CO and H2, which are subsequently used for ethylene hydroformylation catalyzed by the nearby Pt-SiO2 interface. Consequently, propanal can be produced selectively from methanol and ethylene on the nanocrystal bilayer catalysts.
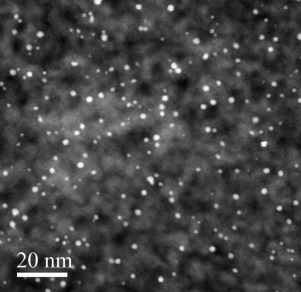
Supported Pt nanoparticle catalysts were synthesized by the particle ALD process. A cross-sectional scanning transmission electron microscopy (STEM) image of Pt/SiO2 is displayed in Figure 1. The average Pt particle size was about 2 nm. An attempt was made to coat an ultra-thin layer of CeO2 (55 cycles of CeO2 ALD) on Pt/SiO2 surfaces. For comparison, Pt nanoparticles were also directly coated on CeO2 particles.
Figure 1. Cross-sectional STEM image of porous silica gel particle supported Pt nanoparticles synthesized by ALD.
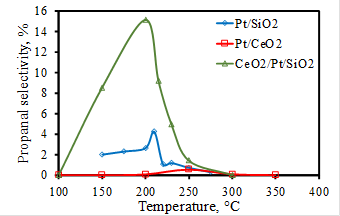
These three catalysts (Pt/SiO2, Pt/CeO2, and CeO2/Pt/SiO2) were employed to catalyze the ethylene hydroformylation reactions. Ethylene, CO, and H2 with a total flow rate of 14 sccm (C2H4: CO: H2 = 1: 3: 3) were introduced into a fixed bed reactor. The CeO2/Pt/SiO2 showed the highest activity and propanal selectivity. As shown in Figure 2, the highest propanal selectivity reached at 200 °C, but the ethylene conversion was only 2.7%. When the ethylene conversion increased at a higher temperature, the propanal selectivity decreased to a pretty low value. Therefore, the yield of propanal was very low. Further experiments are needed to verify this concept and improve the performance of the catalysts.
Figure 2. Propanal selectivity of ethylene hydroformylation reactions.
We also prepared Ni nanoparticles that were supported on porous gamma-alumina particles by ALD. The catalysts were evaluated for a DRM reaction. Through a DRM reaction, both methane and carbon dioxide (main greenhouse gases) can be converted into syngas. The H2/CO ratio is always lower than 1 due to a reverse water-gas shift reaction (CO2+ H2 ⇌ CO + H2O), which is suitable for the Fischer-Tropsch process to produce more high-value C5+ chemicals. To more conveniently observe Ni nanoparticles, Ni nanoparticles were deposited on nonporous alumina nanoparticles by ALD. Small Ni nanoparticles (~3 nm) were also uniformly deposited on the alumina nanoparticles (Figure 3).
Figure 3. TEM image of Ni nanoparticles deposited on nonporous alumina nanoparticles by ALD.
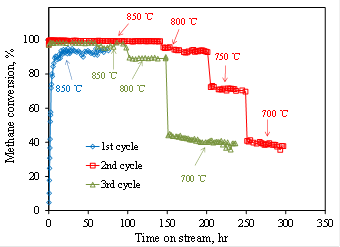
The ALD-prepared Ni catalyst showed very high activity and stability (Figure 4). The experimental details and results are published in Applied Catalysis B: Environmental (Appl. Catal. B., 201, 302-309, 2017).
Figure 4. Methane conversion of dry reforming of methane catalyzed by porous ALD Ni/γ-Al2O3 catalyst at different temperatures.
In summary, this ACS-PRF grant has allowed us to complete the synthesis and testing of various metal nanoparticle catalysts by ALD. The findings and experiences resulting from this funding helped us develop worthwhile projects that have expanded the scope and significance of the initial project supported by ACS-PRF. For example, a catalyst for DRM reaction has been one of the focuses in our lab. In the following year, we will keep working on the CeO2/Pt/SiO2 catalyst and explore the potential for additional applications of ALD prepared catalysts. One PhD student, partially supported by this grant, graduated in May 2016.