Reports: UNI154964-UNI1: Synthesis of Heterocycles by Palladium-Catalyzed [2+2+2] Cyclizations
Seann P. Mulcahy, PhD, Providence College
Transition metals have a primary role in the synthetic organic chemist's toolbox. Many functional groups have an affinity for one or more transition metal, and the resulting complexes often change the reactivity of the functional group. By taking advantage of this new reactivity, chemists are able to perform transformations that were once costly, time-consuming, or resulted in significant waste generation. Our group has been engaged in a research project that uses palladium-catalyzed activation of triple bonds – alkynes and nitriles – to forge the construction of nitrogen-containing heterocycles. We hypothesized that palladium could activate pi bonds in alkynes and nitriles in order to facilitate a [2+2+2] cyclization reaction to form substituted pyridines. Our preliminary work established an unprecedented, yet critical role for palladium in this reaction, and we are using ACS PRF funds to widen the substrate scope of this methodology at a primarily undergraduate institution. Thus, our primary objective is to establish an undergraduate research program that facilitates heterocycle formation through palladium-catalyzed activation of alkyne and nitrile substrates. Towards this goal, we developed two specific aims, the first of which was the major focus of the first year of the funding period.
Aim 1: Develop a fully intramolecular [2+2+2] cyclization that will afford annulated pyridoindoles that are isomeric in structure.
Aim 2: Establish conditions for a partially intramolecular variant, which will yield annulated isomeric pyridines.
The impetus for Aim 1 came from our initial discovery that the diynyl nitrile substrate 1 cyclizes rapidly to annulated pyrido[3,4-b]indole 2 in excellent yield (Figure 1) in the presence of Pd(PPh3)4. Seeking to increase the substrate scope of this methodology, we used a three-step sequence to prepare functionalized diynyl nitrile substrates that were capable of this same [2+2+2] cyclization.
Figure 1.
Palladium-catalyzed [2+2+2] cyclization of a diynyl nitrileTable 1 shows the diversity of diynyl nitrile substrates 7a-k we have generated using this methodology. These substrates bear various functional groups on the aniline ring, as well as a diversity of chain lengths and atom compositions in the alkynyl nitrile tether. The synthesis of these substrates proceeded in moderate to excellent yields.
Table 1. Synthesis of diynyl nitrile substrates 7a-k
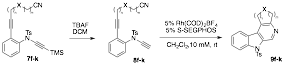
With substrates 7a-k in hand, we began studying the key palladium-catalyzed [2+2+2] cyclization reaction to yield annulated pyrido[3,4-b]indoles, similar to 2. Surprisingly, many of these substrates reacted very poorly. For example, substrates 7c, 7d, and 7e gave 34%, 29%, and 33% yield, respectively (compared to 91% for 2). We are currently trying to optimize these conditions by using more active phosphine ligands. During this investigation, we noticed that we could generate the annulated pyrido[3,4-b]indoles directly from the iodoaniline substrates 5 in low yield. This one-pot Sonogashira/desilylation/[2+2+2] cyclization proceeded for 7a (16%), 7c (33%), 7d (11%), 7j (36%), and 7k (31%) when two portions of 5 mol % Pd(PPh3)4 was used. While much optimization is needed, this demonstrates that a one-pot procedure is synthetically viable, enabling pyrido[3,4-b]indoles to be created in as few as three steps.
With the understanding that the palladium-catalyzed reaction would require extensive optimization, we attempted to demonstrate that the desired pyrido[3,4-b]indoles could be prepared by more conventional rhodium(I) catalysis. Table 2 shows the additional two-step scheme for the formation of annulated pyrido[3,4-b]indoles 9f-k under Rh(I)-catalysis. The substrate scope for this sequence is tolerant to many functional groups and does not suffer from any entropic cost with the formation of a seven-membered ring annulation.
Table 2. Substrate scope for the Rh-catalyzed construction of annulated pyrido[3,4-b]indoles
Substrate
| Yield of 8
| Yield of 9
|
7f
| 89
| 84
|
7g
| 76
| 71
|
7h
| 65
| 77
|
7i
| 83
| 75
|
7j
| 75
| 38
|
7k
| 88
| 41
|
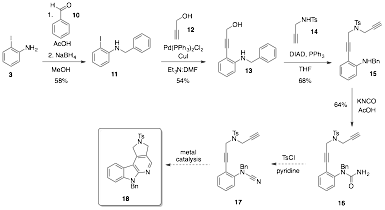
Finally, we have begun to explore the synthesis of an annulated pyrido[2,3-b]indole via similar methodology. Our goal again is to use palladium for the [2+2+2] cyclization, but we recognize that a cyanamide may behave differently than an aliphatic nitrile, so other metals will be explored. Figure 2 shows the synthesis of urea 16, which we hope to convert into the pyrido[2,3-b]indole 18 in two steps. While fairly linear, many of these reactions have been performed on gram scale in good yield. This synthesis has the advantage that aryl or heteroaryl groups could be introduced via a Sonogashira reaction of substrates 15 or 16 (even 17) in order to introduce additional diversity at a late stage in the overall scheme.
Figure 2. Synthesis of a pyrido[2,3-b]indole
In the next year, we will finish our optimization of the palladium-catalyzed [2+2+2] cyclization of substrates 7a-k, prepare diverse diynyl nitrile substrates for the synthesis of annulated pyrido[2,3-b]indoles, and begin to explore an intermolecular variant of this methodology (Aim 2). We are happy to report that we have secured additional funding from the National Science Foundation's Research at Undergraduate Institutions mechanism in the Chemical Synthesis (SYN) program of the Division of Chemistry (CHE), starting September 1, 2016 (grant number 1565987).