Reports: UR453166-UR4: Solid-State Chemistry of the Nitrile Oxides
William Hubert Ojala, University of St. Thomas
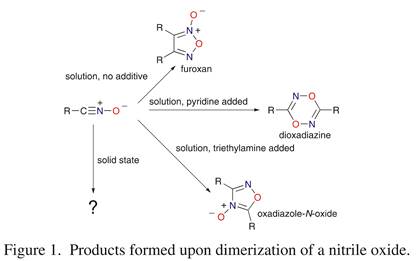
The solution-phase chemistry of nitrile oxides, compounds of formula R-CºN+_O- , is well known by virtue of their common use in synthesis. We have recently completed our third summer undergraduate research season in our study of the solid-state chemistry of these compounds. In solution, nitrile oxides dimerize; the product depends on the reaction conditions: a furoxan (absence of added catalyst), a dioxadiazine (presence of added pyridine), or an oxadiazole-N-oxide (presence of added triethylamine) (Figure 1). We are investigating: (1) How readily do nitrile oxides dimerize in the solid state? (2) Does the identity of the solid-state dimerization product depend on the molecular packing arrangement of the parent nitrile oxide, different polymorphs of the same nitrile oxide yielding different dimers? (3) Does a solution-phase reaction of sterically hindered nitrile oxides, rearrangement to the isocyanate, occur in crystals of these same nitrile oxides on heating? (4) Can nitrile oxides be co-crystallized with structurally similar nitriles, and can solid-state cycloadditions occur that yield products different from those formed from solution-phase cycloadditions? To address these questions, we
synthesize nitrile oxides and their dimers and determine their crystal structures by single-crystal X-ray diffraction. These efforts have been made possible by the support of the ACS-PRF in the form of funding for undergraduate student research stipends and for data collection at the X-ray Crystallographic Laboratory of the Chemistry Department of the University of Minnesota. Data sets we collect at the XCL are transferred to our undergraduate laboratory in the Chemistry Department of the University of St. Thomas, where the structures are refined and analyzed using the SHELX, Mercury, and PLATON program packages.
Because their relative stability facilitates their handling, we focus on benzonitrile oxides and their dimers. To establish an experimental means of determining which dimer(s) might be produced upon solid-state reaction, we prepare authentic samples of the dimers from solution and characterize them by both X-ray diffraction and infrared spectroscopy. In this current reporting period we have determined the crystal structures of compounds representing all three structural families possible to obtain upon dimerization (Figure 2). Among the furoxans, we have determined the crystal structures of two polymorphs of the bis(4-methylphenyl)furoxan as well
as the crystal structures of the bis(3-chlorophenyl)furoxan and the bis(3-nitrophenyl)furoxan. Among the dioxadiazines, we have determined the crystal structures of the bis(4-methylphenyl)dioxadiazine and the bis(4-bromophenyl)dioxadiazine. Among the oxadiazole-N-oxides, we have determined the crystal structure of the bis(2,6-dichlorophenyl)oxadiazole-N-oxide. We also determined the crystal structure of the bis(4-nitrophenyl)oxadiazole, which has been reported to form upon reaction of the initially formed oxadiazole-N-oxide with excess starting benzonitrile oxide.
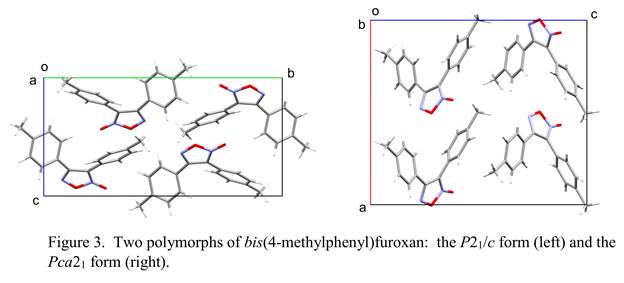
Among our furoxans, we are especially interested in the polymorphism shown by the bis(4-methylphenyl) derivative (Figure 3). In earlier work, we determined the crystal structures of two polymorphs of the corresponding bis(4-chlorophenyl)furoxan, a molecule with similar
space-filling requirements. X-ray analysis indicated that the orthorhombic form (space group Pbcn) of this compound undergoes a solid-state phase transformation to the monoclinic form (space group C2/c) on heating. We have now recently observed a phase transformation in the monoclinic form (space group P21/c) of the bis(4-methylphenyl) analogue. It is of interest to us that these non-isomorphous crystals of structurally similar molecules both undergo solid-state phase transformations. Our next task is to identify the product of this transformation in the bis(4-methylphenyl) analogue by single-crystal X-ray diffraction to determine whether it is in fact the orthorhombic form (space group Pca21) we have prepared by crystallization from solution or is instead a new, third polymorph.
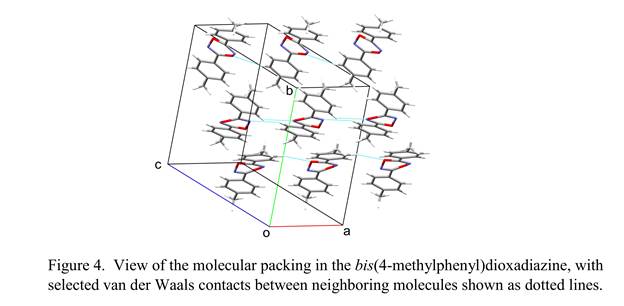
The central ring of our dioxadiazine structures is severely nonplanar, as shown by our bis(4-methylphenyl) derivative (Figure 4). This ring also shows strong indications of positional
disorder (different conformations of the ring) and occupational disorder (exchange of nitrogen and oxygen atoms). Both structures will require additional refinement to establish a satisfactory disorder model. Crystal disorder occurs also in most of our furoxan structures, with the molecule assuming two different orientations related by twofold rotation about its near-twofold axis; this results in two possible locations for the exocyclic oxygen atom, one on each side of the five-membered ring. (In Figure 3, only the major component of the disorder is shown.)
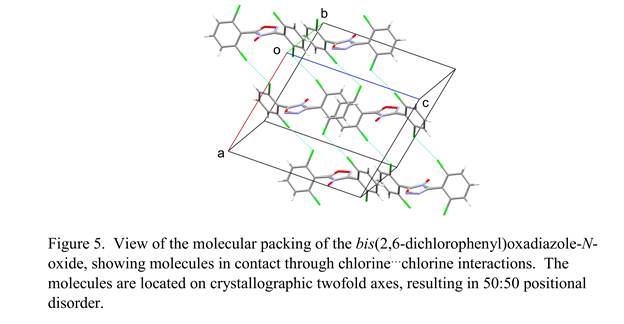
Disorder is also found in our bis(2,6-dichlorophenyl)oxadiazole-N-oxide structure (Figure 5). Each molecule is located on a crystallographic twofold axis but lacks twofold symmetry, so 50:50 disorder about the axis is present, exchanging the N-C=N and N=C-O
moieties of the five-membered rings. The molecular packing arrangement features chlorine…chlorine contacts that connect the molecules into ribbons extending through the crystal. Similar disorder about a twofold axis is found in our bis(4-nitrophenyl)oxadiazole structure (Figure 6). Both crystal structures await further refinement in order to establish a completely satisfactory model for this disorder.
Our ongoing work will include further examination of benzonitrile oxides and their dimers, including polymorphism studies to determine the influence of benzonitrile oxide crystal structure on solid-state dimerization. Our initial attempt to co-crystallize benzonitrile oxides with their corresponding benzonitriles (using 2,6-dichlorobenzonitrile oxide and 2,6-dichlorobenzonitrile) for solid-state cycloadditions proved unsuccessful, but we intend to continue those efforts. All of our crystal structures are of publication quality and have not been published by previous workers; it is our goal to publish them not as individual structure reports but in a comprehensive paper. In the meantime, we have presented this work in a variety of venues, with three members of our undergraduate research group presenting posters at the Spring 2016 National Meeting of the American Chemical Society in San Diego, and five members of our group presenting posters at the August 2016 Summer Undergraduate Research Expo sponsored by the Materials Research Science and Engineering Center (MRSEC) of the University of Minnesota—Twin Cities. This work was also the basis of my poster presentation at the June 2016 Gordon Research Conference on Crystal Engineering. It has been the support of the American Chemical Society Petroleum Research Fund that has made these positive results possible for me and for my undergraduate students.