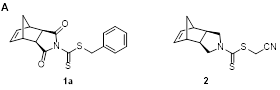Reports: DNI754884-DNI7: Tapered Bottlebrush Polymers: A New Polymer Topology
John B. Matson, PhD, Virginia Polytechnic Institute and State University
Bottlebrush polymers are unique macromolecules comprised of polymeric side-chains densely grafted to a polymer backbone. The highly branched nature of bottlebrush polymers leads to steric repulsion between polymeric side chains, forcing these macromolecules to adopt a chain-extended conformation. As a result, bottlebrush polymers have unique mechanical properties and may find applications in areas including rheology modifiers, super-soft elastomers, and photonic crystals, among others. Our long-term goals are to explore new synthetic methods to make bottlebrush polymers and apply them to synthesize cone-shaped versions of the polymers. This report focuses on our synthetic efforts, detailing our work on three synthetic efforts: 1) Developing new chain transfer agents (CTAs) for use in reversible addition–fragmentation chain transfer (RAFT) polymerization to make macromonomers; 2) Executing a one-pot method for bottlebrush polymer synthesis; and 3) Establishing the importance of the ‘anchor group’ in ring-opening metathesis polymerization (ROMP) synthesis of bottlebrush polymers by the grafting-through technique.
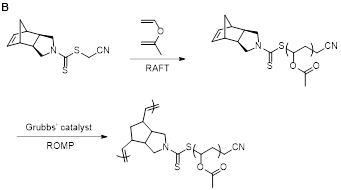
In the first project, two new dithiocarbamate chain transfer agents (CTAs) with norbornene-containing Z-groups were prepared for use in RAFT polymerization. This work was led primarily by graduate students Scott Radzinski and Jeffrey Foster, but most of the experiments were carried out by undergraduates (Sally Lewis and Matt Slutzker). First, CTA 1, a new compound, was prepared (Figure 1A). It effectively mediated RAFT polymerization of 2° more activated monomers (MAMs) but could not mediate RAFT of 3° MAMs or less activated monomers (LAMs). A related compound, CTA 2, was also prepared. It effectively mediated polymerization of LAMs, but did not control RAFT of MAMs. To demonstrate the utility of these new CTAs, poly(vinyl acetate) (PVAc), was prepared by RAFT polymerization mediated by CTA 2 (Figure 1B). ROMP was the performed to form a bottlebrush polymer, which achieved full conversion within 20 min, resulting in the formation of a well-defined PVAc bottlebrush polymer with a narrow molecular weight distribution. This work represented the first synthesis of a bottlebrush polymer with PVAc side chains by the grafting-through method. We expect that this work will be significant in will assist in the rational design of CTAs capable of mediating RAFT polymerization to form polymers functionalized with ROMP-active norbornene moieties.
Figure 1. A) Structures of CTA1 and CTA2. B) Synthesis of bottlebrush PVAc by RAFT and ROMP grafting-through. |
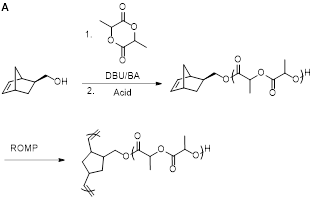
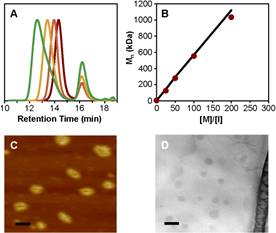
In the second project, bottlebrush polymers were synthesized using a tandem ring-opening polymerization (ROP) and ring-opening metathesis polymerization (ROMP) strategy. ROP and ROMP were conducted sequentially in the same pot, affording bottlebrush polymers with molecular weights in excess of 106 Da. In the first step a polylactide macromonomer (MM) was synthesized via ROP of d,l-lactide initiated by an alcohol-functionalized norbornene. Immediately after quenching the ROP reaction, Grubbs’ 3rd generation catalyst was added to initiate ROMP grafting-through in the same pot to produce the bottlebrush polymer. This methodology was examined for different MM molecular weights and bottlebrush backbone degrees of polymerization. Excellent molecular weight control was attained over both polymerization steps. Bottlebrush polymers were imaged using atomic force microscopy (AFM) and transmission electron microscopy (TEM) using a stain-free technique on graphene oxide.
B |
Figure 2. A) Synthesis of polynorbornene-graft-polylactide bottlebrush polymers using a one-pot approach. B) Characterization data on these polymers, showing polymerization kinetics, control over molecular weights, and imaging (AFM and TEM). |
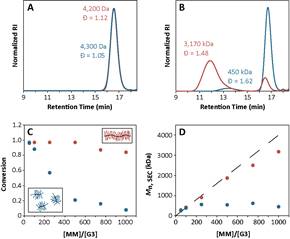
In the third project, we evaluated the effect of the anchor group (the configuration of atoms linking a polymer to a polymerizable norbornene) on the kinetics of ROMP of various macromonomers initiated by Grubbs’ 3rd generation catalyst. A 4-fold difference in the rate of propagation between similar macromonomers with different anchor groups was measured. This phenomenon was conserved across different macromonomers. The observed 4-fold difference in propagation rate affected the maximum obtainable backbone DP (Figure 3). Slowly propagating macromonomers reduced the maximum bottlebrush MW by an order of magnitude (from ~106 Da to ~105 Da). Experimental and computational studies indicated that the rate differences arise from a combination of varying steric demands and electronic structure among the various anchor groups. This work shows that rational selection of the anchor group is critical to achieve high macromonomer conversion in ROMP. It will allow us and other groups to prepare pure, high MW bottlebrush polymers by ROMP grafting-through.
Figure 3. ROMP grafting-through with increasing [MM]/[G3] ratios for two macromonomers with equivalent polymers but different anchor groups (red and blue). A) SEC traces of both MMs. B) SEC traces of bottlebrush polymers resulting from ROMP of both MMs with target DP=1000. C) Conversion (as measured by SEC) vs target DP for both MMs. D) Mn as a function of target DP for both MMs. |
The impact of this award on graduate and undergraduate students at Virginia Tech has been substantial. Two graduate students (Jeff Foster and Scott Radzinski) have been funded in part on this grant. In addition, seven undergraduates (Sally Lewis, Matt Slutzker, Samantha Scanneli, Karen Tran, Kyle Moran, Eric French, and Kearsley Dillon) have contributed to this project. As noted above, for the first project the undergraduate students carried out many of the experiments under supervision from the graduate students. Two undergraduates (Lewis and Slutzker) contributed as co-authors on a paper, and others will contribute as coauthors in future publications.
This grant has also impacted my career. Thus far three papers have been published with support from this award. I presented results from this work at an invited talk at the 2nd Fusion Functional Polymeric Materials Conference in 2015. I have also been invited to speak at several departmental seminars across the country, at which I have referenced this work and this award.