Reports: DNI554840-DNI5: Understanding the Solid-Confined Ionic Liquid Nanofilms
Lei Li, PhD, University of Pittsburgh
Summary
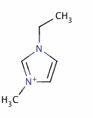
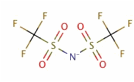
In the past year, we have explored the molecular-level arrangement and simultaneous hydrophilic/oleophobic wetting behavior of a nanometer-thick ionic liquid, i.e., 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide (EMIIm) whose chemical structure is shown in Figure 1, on the silica substrate.
Topography
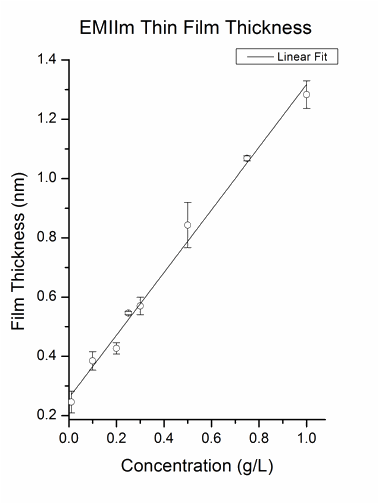
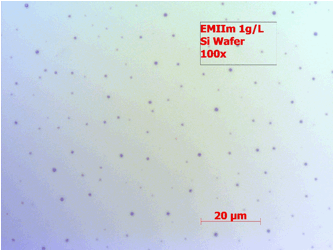
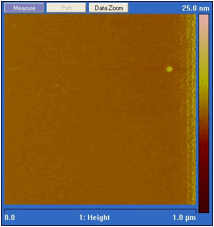
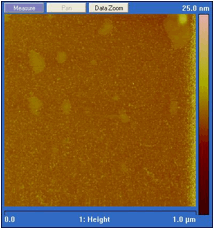
The nanofilm of EMIIm was fabricated by dip-coating and ellipsometry was used to measure the average thickness of the thin film on a silicon wafer. In Figure 2, the film thickness is plotted as a function of solution concentration. There is a positive linear relationship between the solution concentration and the thin film thickness. For further analysis, the monolayer thickness (ML) of EMIIm was determined by converting molar volume to size per molecule, assuming cubic volume arrangement. The ML thickness for EMIIm is estimated to be 0.75 nm. The film topography of three EMIIm/silica samples of increasing thickness was analyzed via optical microscope and atomic force microscopy (AFM). As shown in Figure 3, 0.6 ML sample shows a smooth surface while 1.1 ML and 1.7 ML samples show droplets on the surface. AFM topography images of the same three samples confirm the findings as shown in Figure 4. The AFM of the 0.6 ML sample shows a smooth even film while 1.1 ML and 1.7 ML sample show clear droplets. Optical and AFM results indicate that dewetting occurs when the film is thicker than some critical value, which is between 0.6 ML and 1.1 ML. Below the critical thickness, the EMIIm film is uniform and there is no dewetting. These results can be explained by the trade-off between surface tension of ionic liquid and the liquid-solid interfacial attraction. When the film is thin, liquid-solid attraction, e.g., electrostatic force between silica surface and the EMIIm, dominates and the film is uniform. When the film gets thicker, surface tension of EMIIm becomes more important and the droplets are formed.
Molecular-level Structure
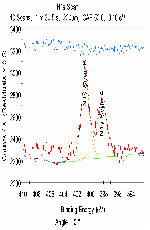
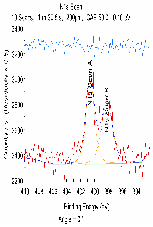
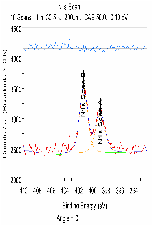
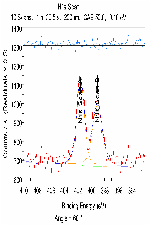
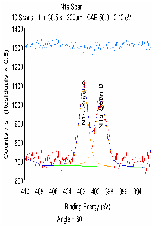
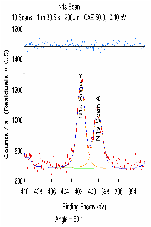
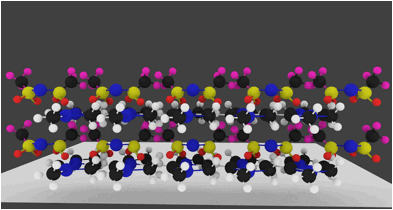
The molecular arrangement of EMIIm on the silica was investigated with angle-resolved x-ray photoelectron spectroscopy (AR-XPS). In bulk EMIIm, the atomic ratio of nitrogen from the anion to nitrogen from the cation (N-/N+) is 0.5. In the nanofilm of EMIIm on the silica, N-/N+ should remain 0.5 if anion/cations have a random arrangement. However, if the anion/cations have a layered structure, the top layer will attenuate the bottom layer and N-/N+ could deviate from 0.5. Moreover, at higher takeoff angles, N-/N+ will further deviate from 0.5 since higher takeoff angle is more sensitive to the top layer. The N1s high-resolution AR-XPS spectra of three EMIIm/silica samples are shown in Figure 5 and the N-/N+ values for all the samples at 0° and 60° takeoff angles are summarized in Table 1. N-/N+ of all three samples is above 0.5, indicating that the anion stays on top of the cation. Moreover, N-/N+ of 0.6 ML sample increases from 0.80 to 0.94 when the takeoff angle increases from 0° and 60°, which also suggests that the anion stays on top of the cation. The schematic of the layered anion/cation/silica structure is shown in Figure 6.
Table 1: ARXPS spectra (N 1s) fitting results for EMIIm thin film
Thickness (± 0.1 ML) |
0.6 |
1.1 |
1.7 |
Atomic Ratio N-/N+ (± 0.01) (0°/60° Takeoff Angle) |
0.80/0.94 |
0.72/0.71 |
0.70/0.77 |
Simultaneous Oleophobicity/Hydrophilicity
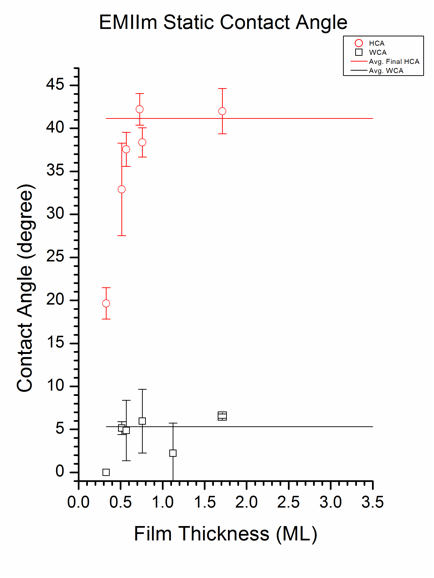
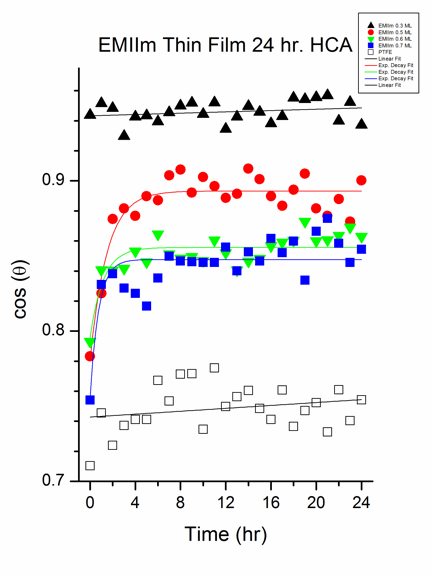
Simultaneous hydrophilic/oleophobic coatings, whose oil contact angle (OCA) is higher than water contact angle (WCA), are highly desirable for various applications, including anti-fogging, oil-water separation and detergent-free cleaning. However, most of the state-of-the-art coatings have higher WCA than OCA. Interestingly, as shown in Figure 7, WCA of EMIIm/silica is ~5.33° (± 3.91°) while the hexadecane contact angle (HCA) plateaued to 41.15° for samples with thickness greater than 0.7 ML, indicating a simultaneously oleophobic/hydrophilic wetting behavior. As also shown in Figure 7, the fact that the HCA increases with the EMIIm thickness up to ~0.7 ML indicates the importance of the film thickness for the wetting with respect to hexadecane. Meanwhile, there is no correlation between the EMIIm thickness and WCA. We hypothesized that the wetting mechanism is dependent on the size of the contact liquid molecule. A water molecule is small compared to hexadecane: the size of a water molecule is ~0.275 nm while the size of hexadecane is ~0.788 nm. As shown in above-discussed AR-XPS study, EMIIm has layered structure on the silica with anion on top of the cation. Since anion has CF3 segments, it renders the top of the EMIIm surface oleophobic. Because hexadecane has larger molecular size, it penetrates the EMIIm, possibly via passing through the inter-molecular “holes”, very slowly and shows higher HCA. Meanwhile, water can pass through the EMIIm film due to its small molecular size and shows small WCA. If this hypothesis is true, HCA should decrease with time since the hexadecane passes through the EMIIm film gradually. To test the hypothesis, time-dependent HCA tests were conducted. A shown in Figure 8, HCA decreases with time and reaches the equilibrium value after ~3 hours, indicating that the hexadecane penetrates the EMIIm film gradually. Meanwhile, such time-dependent HCA is not observed for polytetrafluoroethylene (PTFE). In addition, the final HCA had a positive correlation to the film thickness.
Future Plan
To fully understand the molecular structure of ionic liquid confined to the solids and uncover the mechanism of simultaneous oleophobicity/hydrophilicity, we plan to: (1) investigate various solids, including mica and graphitic carbons. (2) explore the ionic liquids with different cations and anions. (3) Test the anti-fogging performance of ionic liquid coatings.
Figure 1
Figure 2
EMIIm 0.6 ML |
EMIIm 1.1 ML |
EMIIm 1.7 ML |
|
Figure 3
EMIIm 0.6 ML |
EMIIm 1.1 ML |
EMIIm 1.7 ML |
|
Figure 4
0.6 ML 0° |
1.1 ML 0° |
1.7 ML 0° |
0.6 ML 60°
|
1.1 ML 60° |
1.7 ML 60° |
Figure 6
Figure 7
Figure 8