Reports: UR755397-UR7: Employing Light Scattering Analysis and Zeta Potential Measurements to Study the Assembly and Stability of Poly(3-Hexylthiophene) in Binary Solvent Mixtures
David Boucher, PhD, College of Charleston
Recent experimental endeavors have shown that well-ordered poly(3-hexylthiophene) (P3HT) assemblies formed in solution can improve the crystallinity and morphological uniformity of thin films and composites, thereby providing a promising new route to more efficient polymeric optoelectronic materials. Over the past year our group has been conducting fundamental research investigating the use of binary solvent mixtures to direct the assembly of P3HT into hierarchical structures with different sizes, extent of aggregation, and structural order (crystallinity) in the liquid phase. Correlations between the properties of the P3HT assemblies and the solvent blends have been analyzed using solubility parameters, solvatochromic parameters, and COSMO-RS (COnductor-like Screening MOdel for Realistic Solvation) calculations, but with varying degrees of success. In this project we build on our previous work by employing absorption spectroscopy, light scattering and zeta potential measurements to study the stability of P3HT and P3HT assemblies in binary solvent mixtures.
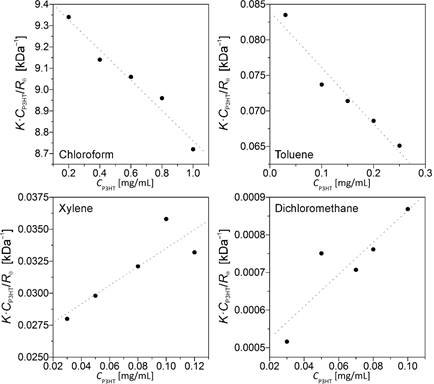
Zeta potential (z) is an important parameter for characterizing the stability of solutions, colloids and aggregative systems. The zeta potential is a measure of the potential difference existing at the interface between the surface of a solid particle immersed in a conducting liquid and the bulk of the liquid. Because the zeta potential depends on the properties of solvent and the surface of the P3HT assemblies, it seems well-suited for investigating correlations between the varying structural characteristics of the assemblies and the solvent environment. Similarly, static light scattering of P3HT solutions and aggregate dispersions at various concentrations, Fig. 1, is employed to determine the value of the second virial coefficient, A2, of P3HT in the solvent.
To date, we prepared a P3HT sample (Mw = 19 kDa and Mn = 14 kDa; PDI = 1.3) using GRIM synthesis. To assess the stability of the P3HT solutions and establish a benchmark for the behavior of P3HT in binary mixtures, we performed baseline solubility measurements of the 19 kDa sample in 29 pure solvents. For comparison, additional solubility measurements were performed on a commercial sample of P3HT (Mn » 30 kDa) using a subset of the 29 solvents. We plan to present the solubility data of the Mn » 19 kDa sample in a manuscript that is currently in preparation. Representative solubility values for the 30 kDa P3HT sample are presented in Table 1 and, as shown in Table 1, we observe good agreement between the static light scattering virial coefficients, A2, and the experimental solubility of 30 kDa P3HT.
Table 1. Solubility, virial coefficients, and computed activity coefficients of P3HT.
Solvent | Solubility (mg·mL–1) | A2 (mL·mol·g–2) |
Chloroform | 14.1 | –9.23×10–4 |
Toluene | 0.71 | –2.61×10–4 |
Xylene | 0.27 | 2.44×10–4 |
Dichloromethane | 0.18 | 2.13×10–3 |
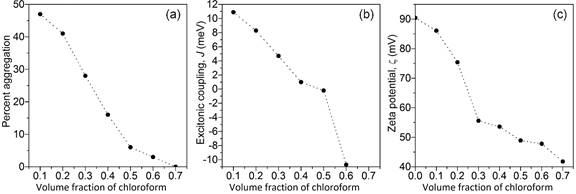
Absorption spectroscopy and zeta potential measurements were performed on 30 kDa P3HT in a series of mixtures of chloroform and dichloromethane (CF:DCM) using 10% volume fraction increments. As shown in Fig. 2(a) and 2(b) the extent (percent) of P3HT aggregation and the excitonic coupling, J, of the P3HT aggregates both increase monotonically with increasing volume fraction of DCM in the solvent blend. The increasing J values reflect the decrease in the structural order of the P3HT aggregates with larger amounts of DCM in the solvent blend. Interestingly, as shown in Fig. 2(c), the zeta potential of the P3HT dispersions in CF:DCM become more positive with increasing DCM composition. The extent of aggregation and the excitonic coupling indicate that the dispersions are progressively more unstable as the DCM composition increases, but based on the general interpretation(s) of zeta potentials the trend in Fig 2(c) contradicts this behavior. Specifically, zeta potentials with larger magnitude(s), positive or negative (±z), signify more stable systems. Typically, colloidal systems are considered unstable if the zeta potential lies in the range between +30 mV and –30 mV. So, although the P3HT dispersions in Fig. 2 may be regarded as having “good stability”, the trend of the zeta potentials in Fig. 2(c) is contrary to the trend expected based on the behaviors in Fig. 2(a) and 2(b). As the extent of aggregation increases, thereby indicating a less stable aggregate dispersion, the zeta potential should move toward zero based on the general criteria used to assess colloidal instability. However, the plot in Fig. 2(b) shows that the excitonic coupling, i.e., structural order, of the P3HT aggregates changes with the composition of dichloromethane.
Within the context of DLVO theory, the molecular level the stability of a colloidal system is determined by the repulsive forces at electrical double layer, VR, and the attractive van de Waals forces, VA, that the particles experience as they approach one another, VTotal = VR + VA. If the properties of the aggregates are the same, or similar, in the series of solvent blends, then perhaps the zeta potentials in Fig. 2(c) would exhibit the expected trend. In this present case, the changing excitonic coupling terms reveal significant variations in the properties of the aggregates in addition to the differences in the extent of aggregation, i.e., coexisting amounts of amorphous and aggregate phases. The anomalous trend in Fig. 2(c) may result from the interplay between these effects, their impact on the nature of the aggregate-aggregate and solvent-aggregates interactions, and the resultant variations in VR and VA related to the observed zeta potentials. Work is currently underway to investigate this supposition in more detail.
During our first summer of research three students were funded by this grant. One student graduated from the college is currently a SULI intern at the NREL in Golden, CO. The other two students are continuing to work on the project throughout the 2016-2017 academic year. This year our work resulted in three student posters at the Celebration of Summer Scholars poster session at the College of Charleston and two student posters at the Southeast Regional Meeting of the American Chemical Society in Columbia, SC. One manuscript has been published with partial support of the donors of the American Chemical Society Petroleum Research. Two manuscripts are currently in preparation and we anticipate that we will be presenting our work at the 252nd National ACS meeting in Philadelphia, PA.