Reports: DNI553648-DNI5: Gasoline Selective Fischer-Tropsch Synthesis in Structured Catalytic Reactors
Georgios Bollas, PhD, University of Connecticut
To prepare the structured catalysts, 200 cpsi cordierite monoliths were shaped to fit a 0.5’’ ID reactor. Alumina wash-coating solution was prepared, by adding 5 g Boehmite and 30 g deionized water into 25 g γ-Al2O3. The monoliths, pretreated at 120 °C, were immersed in the wash-coating solution for 1 min, and the excess solution was gently blown off with pressurized air. After wash-coating, the monoliths were dried at 120 °C for 4 h and calcined at 400 °C for 12 h. Wash-coating was repeated to tune the thickness of the Al2O3 layer. After wash-coating, the active material was deposited by immersing the monolith into a Co(NO3)2•6H2O solution, prepared by dissolving 33.3 g Co(NO3)2•6H2O in 25.6 ml DI water, for 1 min. The excess solution was blown off gently. The catalyst was then dried at 120 °C for 4 h and calcined at 400 °C for 12 h. The final ZSM-5 coating was applied by dip coating the monolith into a NH4-ZSM-5 slurry, prepared by mixing 20 g NH4-ZSM-5 with 31 ml DI water. The excess solution was again blown off, and the previously described drying and calcination protocols were repeated. Two wash-coatings of Al2O3 and a single Cobalt impregnation produced approximately 5 wt.% Co3O4 loading on the monolith. In the following, the notation for the catalyst represents the coating sequence.
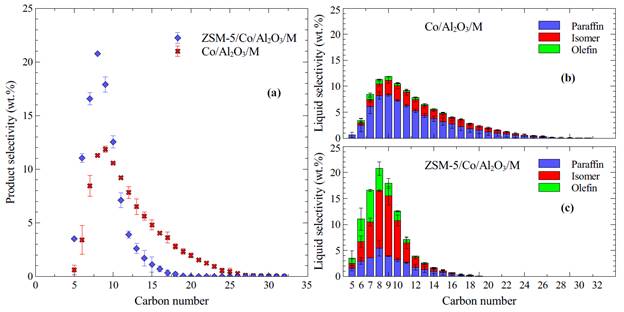
The functionality of the ZSM-5 coating was analyzed in terms of its capacity to enhance cracking and isomerization reactions, as well as its capability to control the size of the hydrocarbons produced. FTS was performed using catalysts with and without ZSM-5 coating to test this hypothesis. Only results that were within 5 wt.% mass balance error were accepted. Experiments were repeated at least three times or as many required to meet the mass balance requirement. The ZSM-5 layer was responsible for decreased CO2 selectivity. The CO2 decrease could be explained by the strong hydrophilicity of the ZSM-5, which decreased the local composition of H2O on the Co sites and limited the extent of the water gas shift reaction over Co. Fig.1 shows that the hydrocarbon selectivity of the C5-C12 range product increased significantly with the ZSM-5/Co/Al2O3/M catalyst. C5-C12 liquid product selectivity reached 60.9 wt.%. The reason for this selectivity improvement is the cracking, isomerization, and oligomerization reactions over the ZSM-5 catalyst membrane. Hydrocracking and isomerization reactions shifted the selectivity of heavy hydrocarbons (>C12) to lighter products (C5-C12), while oligomerization reactions decreased the C2-C4 yields and favored products in the desired C5-C12 range. The Co/Al2O3/M catalyst was selective to heavy hydrocarbons up to C28. There was a shift of selectivity from heavy hydrocarbons to light hydrocarbons when the ZSM-5 coated catalyst was used. The selectivity peak for the ZSM-5/Co/Al2O3/M catalyst was at C8, which indicates a high octane rating and a high-quality gasoline product. The same conclusion can be drawn from Fig.1(b-c). The selectivity to isomers reached 49.8 wt.%, which is about 15 wt.% higher than previously reported values for FTS. Hydrocracking and isomerization over the ZSM-5 coated catalysts were very extensive. In summary, monolith catalysts coated with ZSM-5 membrane showed high activity and high selectivity towards gasoline range products.
Fig.1: Liquid hydrocarbon distribution. (a) Selectivity of different carbon number species for Co/Al2O3/M and ZSM-5/Co/Al2O3/M. (b) (c) Paraffin, isomer, olefin selectivity as a function of carbon number for Co/Al2O3/M and ZSM-5/Co/Al2O3/M.