Reports: DNI954912-DNI9: The Role of Phase Behavior in Fischer-Tropsch Product Distributions: Supercritical Fluid Studies
Michael Timko, PhD, WPI
Objectives. The main objective of this work was the study of the influence of phase behavior on heterogeneously catalyzed chemical reaction. A microreactor was designed and built for in situ investigation. This progress report is an update on phase behavior and in situ studies.
Microreactor Progress. In continued collaboration with our partners at ICMCB (Bordeaux, France), we have designed and fabricated a silicon-pyrex microreactor for in situ studies. In early tests, we have found that the pyrex window limits our maximum operating pressures to approximately 10 bar. We are continuing to work with ICMCB and others to 1) identify thicker pyrex materials to provide a more consistent pressure seal and 2) In the meantime, the PRF award has catalyzed a collaboration on the use of microreactors for studying phase behavior and mixing. We have already published one paper on the subject (albeit on an application tangential to the PRF award and not directly supported by it). And, we anticipate a follow-on paper (which has been supported in part by PRF) to be submitted in 2016 or early 2017.
Phase Behavior & Reaction Engineering. Initially, we intended to study the Fischer-Tropsch synthesis (FTS) reaction as a model for examining the interaction between phase behavior and heterogeneous catalysis. Delays in implementation of safety protocols – specifically for CO – and realization of the challenges of delivering high precision flows of high pressure gases (CO and H2 at 100 bar) to the flow reactor led us to instead use liquid phase dehydration and cracking reactions as our primary model systems. As with FTS, solid acid catalyzed reactions are extremely important in the oil and gas industry; in fact, fluidized catalytic cracking may be the single most important application of heterogeneous catalysis. And, like supercritical FTS, liquid phase reactions catalyzed by solid acids have many interesting features: 1) most importantly for this work, the reactions are heterogeneous, and with potential phase behavior limitations and 2) operation in or near the phase separation boundary may have benefits in terms of process intensity and catalyst lifetime.
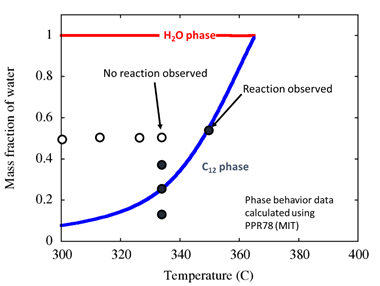
First, we investigated batch-reactor dodecane cracking, catalyzed by ZSM-5 and in the presence of a liquid or supercritical water phase. The reaction mixture was analyzed using GC for conversion, yield, and selectivity. The ratio of dodecane/water and the reaction temperature were varied, at constant pressure of 240-260 bar – a range selected to ensure a liquid (rather than vapor) water phase. Figure 1 shows final results; here, solid dots represent conditions where dodecane conversion was greater than 5%, while open dots correspond to conditions where dodecane conversion was less than 5%. The water-dodecane phase envelope is superimposed on the conversion data for reference. Strikingly,
Figure 1. Effect of phase behavior on observed activity of ZSM-5 for dodecane cracking in the presence of liquid (T < 374 °C) and supercritical (T > 374 °C) water.
Our work with dodecane cracking suggested follow-on studies in a flow reactor. Here, we selected ethanol dehydration as a model reaction and again ZSM-5 as the model catalyst. Unlike the dodecane-water system, ethanol and water are highly miscible. However, the product – ethylene – is immiscible under many conditions. Our first set of experiments with ethanol dehydration revealed interesting behavior. Specifically, we performed experiments to investigate the role of phase behavior by alternating between vapor phase and liquid phase conditions. When vapor phase conditions were investigated – prior to operation in the liquid phase – high ethylene yields were observed. Subsequent operation in the liquid phase dropped the yield significantly and the original activity was never recovered in the
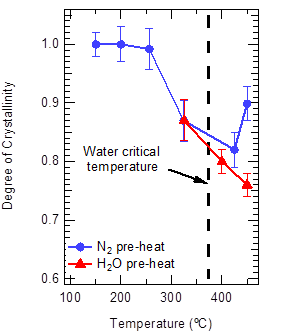
To test the hypothesis that ZSM-5 degradation in the liquid phase was responsible for the observed behavior, we examined ZSM-5 stability under non-reacting conditions. Using crystallinity as our main measurement, we found that ZSM-5 indeed degrades in liquid water, but that the rate is insufficient to explain the rapid (near instantaneous) loss of activity observed for ethanol dehydration under flow conditions. Figure 2 summarizes the results, showing that ZSM-5 retains >80% crystallinity after 3 hours of exposure to hot liquid water. However, we discovered a remarkable phenomenon: ZSM-5 is more stable in supercritical water than in the liquid. Follow-up experiments using gas sorption, SEM, TEM (with collaborators at University of Toronto), MAS-NMR (with collaborators from Clark University), DRIFTS, and XPS strongly suggest that the low ion content of supercritical water can explain the remarkable stability behavior we have observed.
Figure 2. ZSM-5 stability data in condensed water phases, showing that the material is more stable in supercritical water than in liquid water.
Continuing Work. We are continuing our work on ZSM-5 stability in liquid phase water and plan to merge our work with reaction chemistries in bench-scale reactors with our progress in the silicon-pyrex microreactor.
Products. To date, the award has resulted in the following products:
· A.R. Maag, M. Timko, “Liquid-Phase Stability of ZSM-5: Role of De-Alumination and De-Silication”, presented at AIChE (Salt Lake City, UT), November 2015, New England Catalysis Society (Providence, RI), May 2016, and Gordon Conference (Holderness, NH), June 2016.
· A. Zaker, M. Timko, “Catalytic Cracking of Dodecane in Supercritical Water”, New England Catalysis Society (Providence, RI), May 2016.
· M. Timko, “Hydrothermal Stability Strategies for Solid Acid Catalysts”, University of Connecticut (invited lecture), May 2016.
In addition, talks and posters have been accepted for presentation at the national meeting of AIChE (San Francisco, November, 2016). A manuscript on zeolite stability is in the advanced state of preparation for submission to ACS Catalysis. Work on dodecane cracking was only partly supported by PRF; we anticipate submitting a related manuscript in Fall 2016 and then a follow-up phase behavior manuscript in 2017.