Reports: ND554519-ND5: Synthesis and Surface Reactions of Organometallic Precursors Tailored for Electron Beam Induced Deposition
Lisa McElwee-White, PhD, University of Florida
D. Howard Fairbrother, PhD, Johns Hopkins University
Introduction
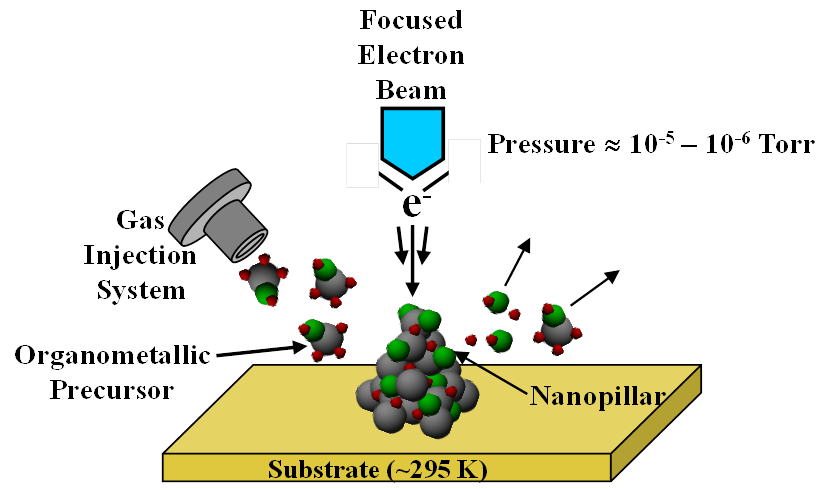
Electron beam induced deposition (EBID) from organometallic precursors is a direct-write vacuum based deposition strategy with the ability to generate spatially and geometrically well-defined three-dimensional, metal-containing structures (Figure 1). Consequently, EBID is uniquely well positioned to create new catalysts across various length scales. However, use of EBID to synthesize metal catalysts requires control over not only the size and shape, but also the chemical composition of the deposits. To date, EBID structures have been created from precursors designed for thermal processes, such as chemical vapor deposition (CVD). However, ligands that dissociate cleanly during CVD are often decomposed by electron induced processes, affording high levels of organic contamination in EBID deposits, which will negatively impact applications. This proposal is focused on the preparation and evaluation of new organometallic precursors designed specifically for EBID.
Figure 1. Schematic representation of a size and shape selected metal-containing structure being deposited by electron beam induced deposition (EBID).
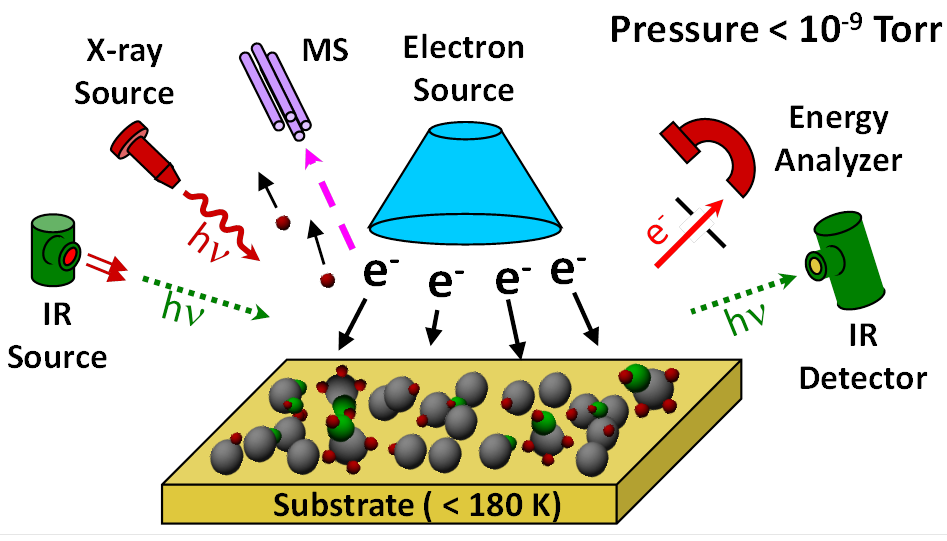
We are using mechanistic information obtained from modelling of the EBID process by complementary gas phase and UHV surface science studies (Figure 2) to provide information useful in formulating design strategies for EBID precursors. In contrast to studies conducted in electron microscopes, where EBID deposits are created under steady state deposition conditions, the UHV surface science approach relies on studying the effect of electron irradiation on nanometer thick films of precursor molecules adsorbed onto chemically inert substrates at low temperatures. Surface analytical tools such as X-ray Photoelectron Spectroscopy (XPS) and Reflection Absorption Infrared Spectroscopy (RAIRS) can follow changes in the surface composition and bonding environment of the various elements within the precursor molecule, complemented by Mass Spectrometry (MS) which can detect the volatile species ejected from the film as a consequence of electron stimulated reactions.
Figure 2. Schematic representation of the Ultra-High Vacuum Surface Science approach to study EBID precursors.
Results and Discussion
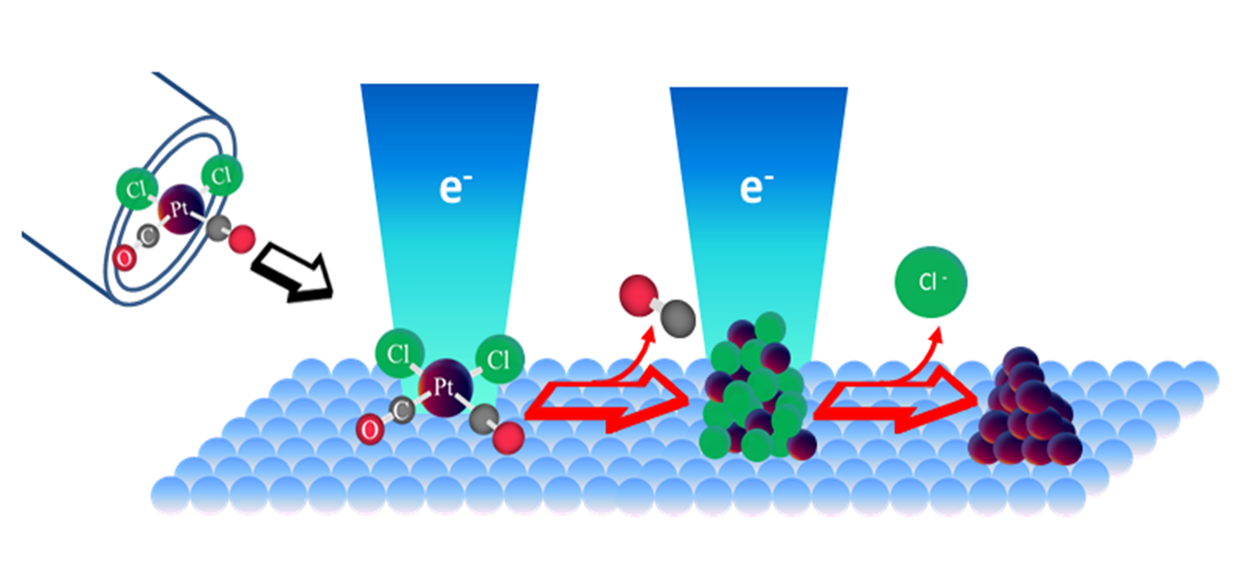
The goals of this project are to evaluate the electron stimulated processes involving ligands that are often present in organometallic precursors and to use the information obtained to design precursors specifically for EBID. In studies on [(η3-C3H5)Ru(CO)3Br] and [(η3-C3H5)Ru(CO)3Cl] during the prior project period, we explored the fate of carbonyl ligands and metal-halogen bonds. The results of these studies led us to target cis-Pt(CO)2Cl2 (1) as a precursor for the EBID of pure Pt nanostructures. We now report mechanistic study of the electron-induced decomposition of 1 under UHV conditions and the deposition of carbon free Pt nanostructures under steady state conditions in an Auger spectrometer. Together these results demonstrate the electron-induced chemistry necessary to obtain pure Pt FEBID nanostructures from 1.
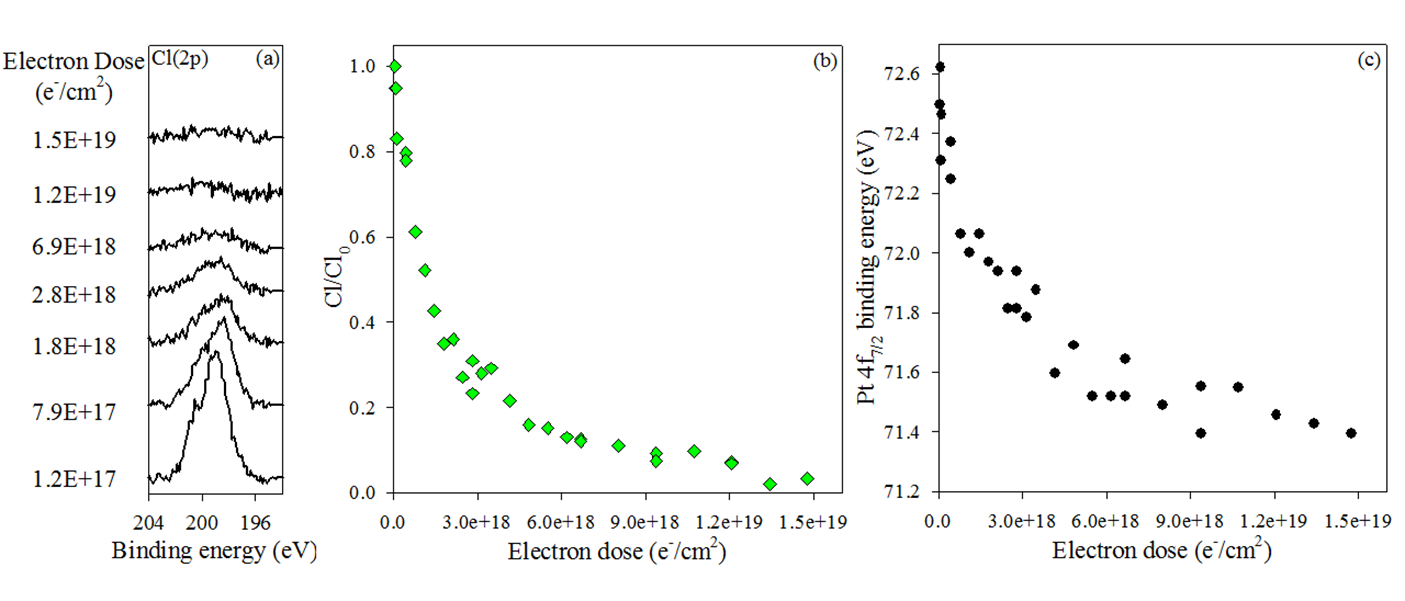
XPS and MS data obtained after electron irradiation in UHV surface science experiments reveal that the surface reactions of adsorbed cis-Pt(CO)2Cl2 (1) proceed in two stages. The initial step involves electron-stimulated precursor decomposition accompanied by the evolution of CO into the gas phase. However, under the influence of more prolonged electron beam irradiation, the film that forms as a result of the decomposition of 1 loses Cl atoms (Figure 3). The overall effect of electron irradiation on 1 is shown in Scheme 1.
Figure 3: a) Cl(2p) XP region for a ~1.3 nm cis-Pt(CO)2Cl2 film adsorbed on a:C, exposed to electron doses ranging from 1.2 x 1017 to 1.5 x 1019 e-/cm2 and corresponding changes in, b) the fractional coverage of adsorbed chlorine atoms normalized to the initial chlorine atom coverage (green diamonds), and c) the Pt 4f7/2 binding energy (black circles), each plotted as a function of electron dose.
Scheme 1: Stage 1: Electron stimulated CO desorption from cis-Pt(CO)2Cl2. Stage 2: Electron stimulated desorption of halogens from the residual PtCl2 product from Stage 1.
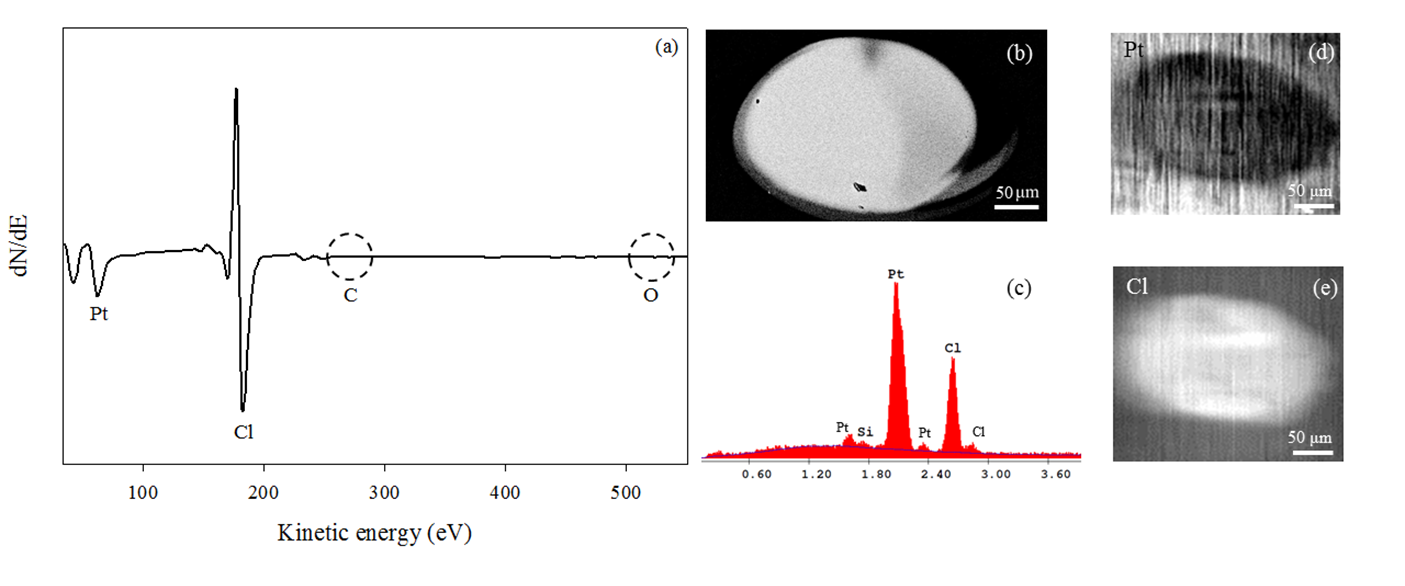
Figure 4 shows Auger electron spectra (AES) for EBID deposits created from 1 on a Ru coated Si/Mo multi-layer substrate under steady state deposition conditions. Formation of the deposit is accompanied by loss of the carbonyl ligands (Figure 4a). In a second step that occurs for significantly larger electron doses, most of the chlorine atoms are removed from the deposits via an electron-stimulated desorption process, analogous to a post-deposition electron-beam processing step (see Figure 3). Coupled with the electron-stimulated removal of chlorine demonstrated in the UHV experiments, the Auger data establish a route to FEBID of pure Pt. In this study, we have demonstrated that mechanistic information from surface science studies of electron-induced reactions of organometallic precursors can be used to successfully identify a precursor for FEBID, supporting the idea that mechanism based precursor design could be broadly applicable to a variety of deposition techniques.
Figure 4. Auger electron and SEM data for a deposit created from cis-Pt(CO)2Cl2 in an AES instrument on a Ru coated Si/Mo multi-layer substrate under steady state deposition conditions (Pcis-Pt(CO)2Cl2 ≈ 1.5 x 10-8 Torr for 19 hours at 3kV, with average target current of 300 nA). The Auger spectrum of the resulting deposit is shown in (a). The secondary electron image of the deposit acquired in a SEM (20kV, 300x) is shown in (b), along with (c) the corresponding EDS data. Auger elemental maps of the deposit and surrounding region are shown for (d) Pt (64 eV) and (e) Cl (181 eV).
Final Remarks
In the prior project period, this project provided the preliminary data the PIs used to obtain industrial funding for related work on EBID precursors. We subsequently used results from this project period to obtain federal funding for additional EBID work. This project also provided excellent training for the graduate students who have participated in this collaboration between PIs in different areas of chemistry at different universities. Three of the graduate students who were involved have obtained their PhDs during the project. Two are employed in the semiconductor industry and the other is an Assistant Professor at an undergraduate institution.