Reports: ND1055283-ND10: Micro/Nanoscopic Investigation of ZnO towards the Development of Next-Generation Separation Platforms
Jong-in Hahm, PhD, Georgetown University
The overall goal of the project is i) to generate well-defined zinc oxide nano and microstructures in the forms of 2D (thin film), 1D (nanorods, microrods), and 0D (nanoparticles, microparticles) and ii) to examine their crystalline facet-specific chemical reactivity for the design of better separation platforms. For this initial funding period, our efforts were focused on the former, well-controlled synthesis of the materials and their characterization.
We have started to work on establishing reliable and reproducible synthetic methods to produce nanostructures and microstructures of zinc oxide in controlled sizes and shapes using both gas- and solution-phase growth approaches. We have developed experimental procedures to produce nanoscale and microscale zinc oxide materials with varying degrees of shape anisotropy. The materials were produced either in a home-built chemical vapor deposition system or in an aqueous solution containing zinc precursors with chemical reagents to selectively control the directionality of the preferential growth of the nucleated crystal.
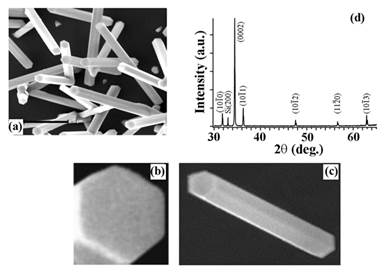
Figure I. SEM images displaying gas-phase synthesized zinc oxide nanorod crystals. The SEM images clearly show the hexagonal basal planes at the two nanorod ends and the rectangular prismic planes on the nanorod main body. XRD data of a typical batch of zinc oxide nanorods display predominant 1D growth characteristics along the c-axis of the nanorods.
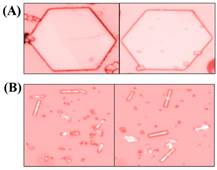
Figure II. Optical micrographs of (A) hexagonal zinc oxide microdisks and (B) zinc oxide microrods grown in aqueous environments using different zinc precursors for controlling the directionality of the preferential zinc oxide growth following nucleation. The dominant crystalline facets of the microcrystals are the hexagonal basal plane in (A) and the rectangular prismic plane in (B).
The various forms of zinc oxide materials were then characterized as-synthesized or after purification by spectroscopic and imaging tools such as SEM, AFM, pXRD, EDAX, ATR/FTIR, UV-Vis, and Raman. The crystallinity as well as the presence of any chemical and physical defects was examined. The surface characterization of the materials was carried out both at the crystal ensemble level using ensemble-averaged measurements and at the individual crystal level employing position-specific analyses along each zinc oxide crystal.
Among the various zinc oxide forms produced, the nanorods were further exploited as an active channel in a photodetector device operating in the visible wavelength range. The zinc oxide nanorod-based devices demonstrated extremely high electrical responses upon light illumination. The outcomes of this work are currently documented in a manuscript.
We have also begun to examine the potentially different degrees of chemical reactivity depending on the crystalline facets present within individual zinc oxide nano- and micro-materials. In addition to the direct imaging methods of AFM and SEM which can provide information on the morphological changes of the crystal surface, we are investigating optical scattering-based method with a sub-crystal spatial resolution to determine position- and crystalline facet-dependent optical responses. An example of this is shown in the figure shown below, which was obtained for the case of elastic scattering from a zinc oxide nanorod. Similar to what is displayed as an example, we are currently in the process of developing a new inelastic scattering signal-based approach which can be exploited for determining the effectiveness of the various zinc oxide materials (sizes, shapes, aspect ratios) serving as a sorbent platform for sulfur.
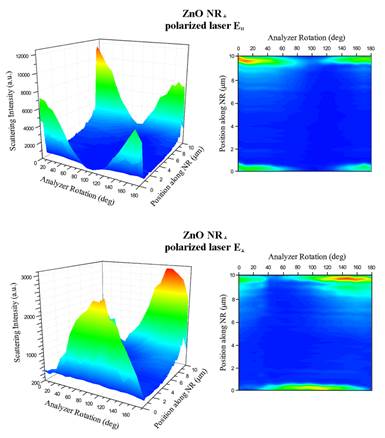
Figure III. Scattering of a single zinc oxide nanorod measured by using two polarization directions of an incoming laser (E║ and E┴) on a nanorod oriented along the x-axis (ZnO NR┴). Scattering intensity was measured with respect to the position along the length of the 1D nanomaterial as well as the analyzer angle. (Top) The 3D contour plot summarizes scattering results from a ZnO NR┴ under the excitation of E║ as a function of both the analyzer angle and the spatial position on the NR. Intense scattering occurred only on the two ends of the NR┴ where the signal along the main body of NR┴ was negligent. This phenomenon is also clearly seen in the 2D projection of the scattering intensity with respect to the analyzer angle at each position along the length of the ZnO NR┴. (Bottom) The same set of scattering measurements was repeated by using the orthogonal excitation of E┴ as a function of both the analyzer angle and the spatial position on the same ZnO NR┴.
A new Ph.D. student has been recruited for the proposed research to continue onto the next phase of the proposed research briefly described above. In the upcoming project period, a preliminary investigation will be launched to quantitatively determine the potential differences in sulfur reactivity when the same reaction is to be carried out on the different crystalline planes of the well-defined zinc oxide nano- and micro-crystals.
Note: A new graduate student, therefore, has been recruited for the proposed research. Matthew Hansen was recruited from a group of incoming Ph.D. students who were accepted to the Department of Chemistry in Fall 2015. While being supported on a Teaching Assistant Fellowship as a first year Ph.D. student, he worked with Daniel to learn the experimental processes involved with the zinc oxide project including the different gas- and solution-phase approaches for producing the well-defined zinc oxide crystals in micrometer and nanometer sizes. In the upcoming project period, he will learn the different characterization techniques for the as-grown zinc oxide nano and microstructures. He will then start on the investigation of the facet-specific H2S activity of the different zinc oxide crystals.
Associated with time involved with the new student recruitment and the period of his mandatory coursework and project training in the laboratory, it is expected that the project timeline to carry out the bulk of the planned evaluation of H2S reactivity will be pushed to 2017. For the immediate future of the next six months, Matt will begin a preliminary investigation and determine quantitatively the reactivity differences on the surfaces of the zinc oxide nano and microcrystals.