Reports: ND554813-ND5: Mechanical and Adhesive Properties of Crude Oil Droplets: Contributions of Interfacial Components
Marjorie Longo, PhD, University of California, Davis
Introduction
Mechanical stabilization of viscous or rigid surfactant films on oil droplets may contribute to kinetically-limited coalescence, thus slowing the separation of oil from water. Fundamental understanding and characterization of this mechanical stabilization with respect to the presence of salts (coions and counterions) is incomplete. In the first year of the grant, we have focused upon characterizing the rigidifying effect of coions and counterions on films of anionic sodium dodecyl sulfate (SDS) and cationic dodecyl amine hydrochloride (DAH) hemimicelles by measuring breakthrough forces using atomic force microscopy. We have found that these films behave similarly to their thicker lipid bilayer cousins, and that AFM is a useful tool for evaluating more film properties than strictly morphology of surfactant films.
Development of Protocols for Breakthrough Force Measurements
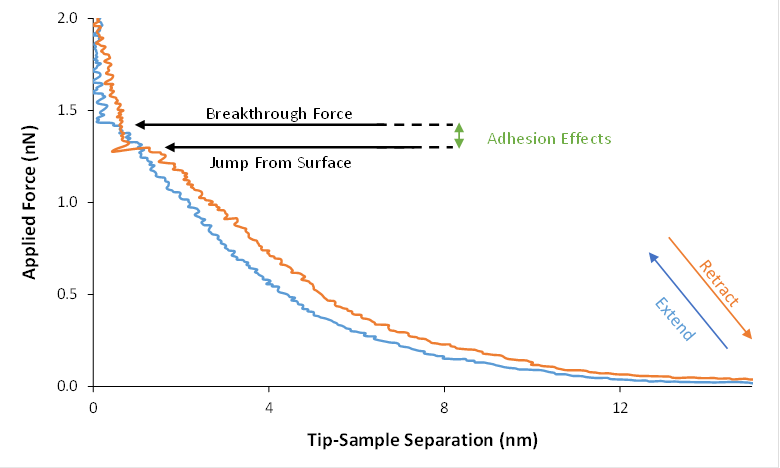
The films were formed by simply preparing a mixture of the desired concentration of surfactant (5-15 mM for SDS or DAH) and allowing it to equilibrate with a freshly cleaved graphite surface for at least 30 minutes. Imaging was done with a Bruker Dimension 3100 using MSCT silicon nitride cantilevers of spring constant 0.05 N/m. Each trial run measuring breakthrough forces requires two important conditions. The first necessary condition is that the same tip be used throughout the run. In order to meet this condition, we changed the concentrations of surfactant and added ions while the tip was still in solution (but away from the surface). The second necessary condition was that the tip remained over the same patch of surface throughout the experiment, as surface conditions can cause widespread variations in breakthrough properties even in the absence of added counterions. Thus, our data for each line was taken in a single continuous trial using the same tip and examining the same patch of surface as small numbers of ions were added to solution. Figure 1 shows an example of a breakthrough force curve from this work.
Figure 1. Example of a breakthrough force curve. This curve was taken from a 10 mM DAH film with addition of 0.26 mM NaCl.
Results
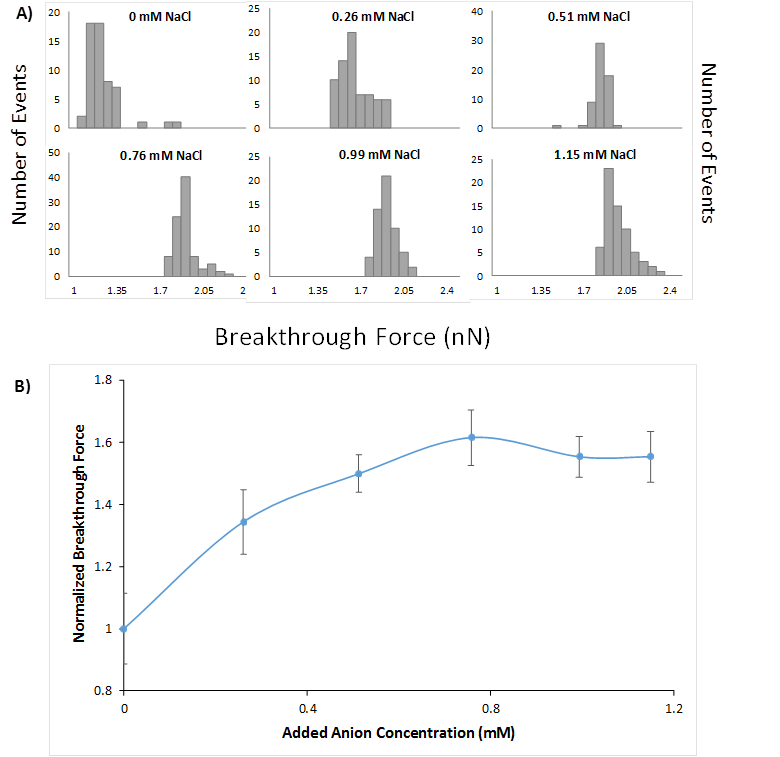
The effect of adding various ions at mM quantities on the breakthrough forces for each surfactant film was clearly discernable by comparing the breakthrough force distributions. As an example, addition of NaCl to DAH caused a distinct shift of the breakthrough force distribution to higher values (Figure 2A) representing a monotonic increase in film strength measured at the mean of each distribution (Figure 2B). The divalent MgCl2 first decreased film strength before causing a significant increase at higher concentrations. In cases where strengthening occurs, the strength of the film approached an upper limit roughly 60-70% greater than that of the initial 10 mM film. We also performed similar measurements by adding H3O+ and OH- counterions to solution. It is important to note that the concentration of these ions corresponds to the bulk value and that concentrations are higher near the film due to the presence of a diffuse double layer.
Figure 2. (A) Breakthrough event distributions for 10 mM cationic DAH films with added NaCl (Cl- is the counterion), (B) Normalized breakthrough forces for 10 mM DAH and added NaCl. Error bars shown represent the width of each sample standard deviation.
Impact
Our work with DAH and SDS surfactant films demonstrate how even small amounts of ions can have dramatic effects upon the mechanical properties of interfacial surfactant films which may contribute to kinetically-limited separation of oil from water.
Educational Impact - Mentoring and Career Development
Training for both graduate and undergraduate students was provided. Students were trained with state-of-the-art atomic force microscopy equipment to make force vs. distance curves and analyze their significance. Two engineering graduate students and one female undergraduate student were supported financially on this grant while they performed this work. One of these graduate students was an under-represented minority who graduated with a PhD at the end of the first year of this grant and began a new postdoctoral position. The other PhD student presented a preliminary version of this work at the American Chemical Society Annual Spring Meeting of 2016 and plans to submit this work for publication in the second year of the grant. In addition, three undergraduate students benefitted from supplies purchased with this grant used for their contributions to this project. Funds from this grant have been crucial for developing a new line of atomic force microscopy investigation for the PI’s laboratory and in restoring the capacity to perform micropipette aspiration measurements planned in the second year of funding.