Reports: ND854498-ND8: Biogeochemical and Lithification Patterns in Microbial Carbonates through Interactions of Sedimentary Bed Forms and Fluid Flow
Tanja Bosak, PhD, Massachusetts Institute of Technology
Taylor Perron, PhD, Massachusetts Institute of Technology
Dr. Giulio Mariotti, a former postdoctoral scholar co-advised by Drs. Bosak and Perron, has established an experimental system to study the biogeochemical properties of and microbial growth on sandy sediments in the presence of bedforms and oscillatory flow. Insights from this system have helped us address four major questions related to the interactions of microbial growth and flow in porous sediments.
1. Traces and textures produced by microbial interactions with siliciclastic sediments
Dr. Mariotti identified interactions between oscillatory flow and cm-scale microbial aggregates that produce elongate trails on the surface of a sediment bed. Such trails abound in late Ediacaran and early Paleozoic sandstones and siltstones and are often attributed to early animals (Fig. 1). Thus, the interaction between flow and microbial aggregates on a sediment bed can produce a number of structures that are currently interpreted as evidence of early animal locomotion (Mariotti et al., 2016).

Figure 1. Comparison between trace fossils (A,B,C,D)
and experimentally created trails (E,F,G,H). Trace fossil images from (A) Mistaken
Point Formation, Newfoundland, Canada, 565 Ma (Liu et al. 2010a); (B) Tacuarí Formation in east-central
Uruguay, >585 Ma (Pecoits et al. 2012); (C) Suz’ma locale, White Sea region,
northern Russia, late Proterozoic (Fedonkin et al. 2007); (D) Puncoviscana Formation, northwest
Argentina, early Cambrian (Buatois and Mángano 2003); Wide and shallow single groove (F)
formed by compact, spherical aggregates with higher sand content. Narrow single
groove with prominent levees (G) and multiple-groove trail (H) formed by
irregular, sand-poor aggregates. All scale bars are 1 cm long.
2. Macroscopic patterns of microbial growth in the presence of rippled sediments and oscillatory flow
Dr. Mariotti also explored the colonization of ripples by photosynthetic mats in the presence of oscillatory flow and identified distinct spatial patterns of microbial growth that depend on the nutrient abundance and interactions between the flow and bedforms (Fig. 2), Similar macroscopic patterns of mat growth on sand ripples might be used to infer water biochemistry, the ages of microbial cover and may lead to differential patterns of microbially-induced lithification in porous sediments. Dr. Mariotti presented these results at the Ocean Sciences Meeting in 2016.
Figure 2. Microbial colonization of and growth on ripples in the presence of oscillatory flow. Left: Troughs in nutrient-rich medium. Right: Crests in nutrient-poor medium. The black and white scale in the right panel is in centimeters.
3. Fossilization of photosynthetic and soft-bodied organisms in sand and silt
Sharon Newman, a graduate student in the Bosak lab, studies how mat-forming cyanobacteria interact with sediments and flow in siliciclastic environments. She experimentally tested the influence of: (1) silica concentrations in seawater, (2) the abundance of fine particles in sandy substrate, and (3) agitation on the extent and composition of coating around sheaths of filamentous cyanobacteria. Her experiments identified microbial trapping of suspended clays as critical for the early fossilization of microbial textures and showed that this process did not require anaerobic conditions or extensive organic degradation. This taphonomic model helps explain the preservation and abundance of various photosynthetic fossils and textures in sandstones and siltstones during the Ediacaran period, and can account for the preservation of similar features throughout Earth history (Newman et al., 2016, Fig. 3). Another manuscript detailing these experiments is currently in review.
Figure 3. Representative SEM images and SEM-EDS spectra of filamentous cyanobacteria and minerals. A: Filaments in the inoculum (day 0), B: Cells grown on beach sand (5 days), C: Cells grown on illite powder (5 days). The EDS spectra of analyzed areas (squares) are located directly beneath the images. Scale bar: 1 μm. Samples were Au-Pd sputter coated.
Sharon is currently investigating the fossilization of soft tissues and the preservation of organic matter in sand and silt using experiments that address the role of microbes, flow and clay minerals (Fig. 4).
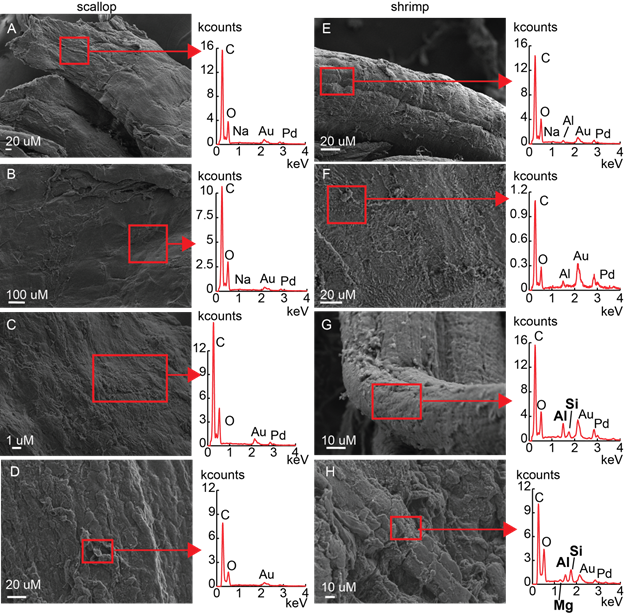
Figure 4. Representative SEM images of soft tissues incubated in the presence of high silica (0.1 mM) and siliciclastic sand. Scallop tissues: (A) microbial mats absent, 20 days, (B) clay-free microbial mats present, 20 days; (C) clay-coated microbial mats present, 20 days; (D) clay-coated microbial mats present, 40 days. Shrimp tissues: (E) microbial mats absent, 20 days, (F) clay-free microbial mats present 20 days; (G) clay-coated microbial mats present, 20 days; (H) clay-coated microbial mats present, 40 days. EDS spectra of areas in squares are to the right of corresponding SEM images.
4. Formation of modern marine ooids
We investigated the role of physical and microbial processes on ooid growth on a beach on the leeward coast of Cat Island, The Bahamas and found that lipids in ooids and grapestones are similar and preserve signals of extensive, and possibly anaerobic, heterotrophic degradation. This revealed a strong contribution of benthic microbes to the organic matter in ooids. We combined field observations and modeling of the sediment transport to suggest that extensive carbonate precipitation occurs around ooids and grapestones in microbially colonized sediments, and that ooids are periodically reworked by storms and transported to the surf zone, where the abrasion can polish their exteriors in less than a day. This new model of ooid accretion makes petrographic predictions that match observations in Cat Island and some other localities (Fig. 5). This work supports a strong role of microbes in the formation of carbonate grains that in porous carbonate sediments, and in the reduction of porosity within microbially-colonized sediments. Dr. Mariotti submitted a paper describing this work for publication in August 2016.

Figure 5. Large (500 µm diameter) ooids from the headland site of Cat Island, The
Bahamas. Note the presence of multiple nuclei (on the left), irregular laminae
(arrow), and the absence of outward thinning of the laminae. These features are
consistent with the predictions of the model developed by Mariotti et al., submitted.