Reports: UR552049-UR5: Synthesis and Multilayering of Poly(4-vinylphenol) Derivatives for Future Applications as Proton Exchange Membranes in Fuel Cells
Ronny Priefer, PhD, Western New England University
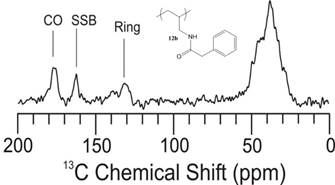
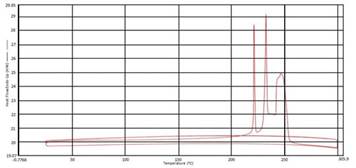
Over the past year, the grant on the design of new multilayer materials as proton exchange membranes for possible application into hydrogen fuel cells has advanced nicely. Three significant advances have been accomplished which led to two separate publications. The first was the synthesis of a library of new pseudo polyelectrolytes (pPE) in particular the alternation of a polyanion. We initially focused on poly(allylamine) hydrochloride (PAH). We looked at performing post-polymerization modification on PAH. We were able to synthesize a library of amide-linked polyelectrolytes with tethered aliphatic, aromatic, and cubyl moieties. Ultimately, we anticipate structure-property relationships observed in the resulting library of graft-modified polymers can facilitate improved understanding of how to design polyelectrolytes for the construction of well-defined multilayer systems. This work also explored the thermal and spectral analysis of these novel amide tethered polymers from PAH. Therefore, we conducted differential scanning calorimetry (DSC), thermogravimetric analysis (TGA), and solid state nuclear magnetic resonance (SSNMR) spectroscopy on all of the polymer samples. From the SSNMR we were able to confirm coupling (Figure 1). None of the polymers displayed a melt profile, but most possessed multiple endotherms (Figure 2). Clean step-wise decomposition was detected from the TGA work (Figure 3a and b). This work was has now been published and we are further exploring the novel polymers in multilayered systems.
Figure 1: Solid State 13C-NMR of polymer phenylacetic acid - PAH-H polymer
Figure 2: DSC Thermogram of acetic acid - PAH-H polymer
Figure 3: Representative TGA curves for 2-chlorobenzoic acid - PAH-H and phenylacetic acid - PAH-H
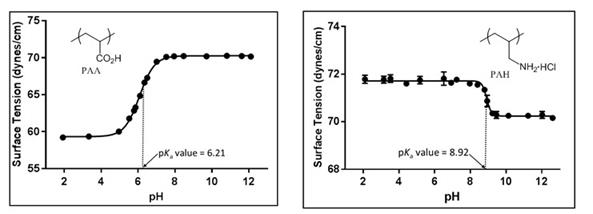
Secondly, we looked at novel ways of determining the pKa values of polymers using surface tension at the surface-to-air interface. Although the determination of pKa values of acids can be done in a multitude of manners ranging from the classic potentiometric titration to computationally, we envisioned that it would be possible to determine the pKa values of polyelectrolytes using surface tension measurements at the surface-to-air interface. When measuring surface tension at the surface-to-air interface of polyelectrolytes at differing solution pH values a classic sigmoidal curve was obtained with the inflection point being the pKa value of the polymer (Figure 4). The pKa value of poly(acrylic acid) (PAA), PAH, poly(ethyleneimine) (PEI), poly(sodium 4-styrenesulfonate) (PSS), and poly(methacrylic acid) (PMA) were all obtained with this novel technique. We were also able to illustrate how both the polymer and salt concentration effects the surface tension at the surface-to-air interface and thus the pKa values. This is the first study of using surface tension at the surface-to-air interface to determine pKa values of acids. This was ultimate published earlier this year. We have expanded this work to small molecules and have seen the same trend. This work still needs to be completed and will hopefully be submitted for publications within a year.
Figure 4: Surface tension of a 0.01M aqueous PAA and PAH solutions at the surface-to-air interface at differing solution pH values.
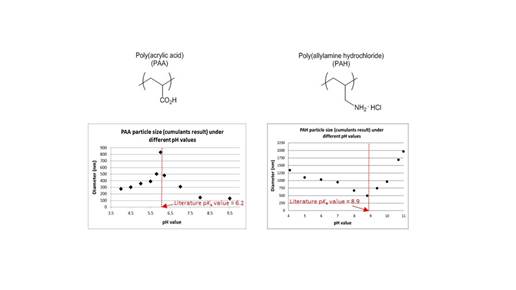
One final area of work which has spun off of the published work highlighted above was the determination of pKa values of polymers using spray dryer in conjunction with particle size analyzer. The spray dryer allows for the careful synthesis of particles of a set size. By formulating solutions of polymers (PAA, PAH, PMA, PEI) at different pH values with identical ionic concentrations while maintaining a set temperature and pressure of the spray dryer, particles should be produced that are pH size dependent. The particles obtained can then be suspended in either tetrahydrofuran or acetone and then measured by a particle size analyzer to determine their size. The primary goal was to see if particle sizes would be different, and if so would they correlate to the pKa value of the polyacids. We can state that there is indeed a correlation between particle size and solution pH values. When we graphed solution pH against particle size, a bell curve shape was determined with a maxima at the polymer’s pKa value (Figure 5). The opposite occurred with poly(allylamine hydrochloride) where there was a bell curve having a minimum at its pKa value (Figure 5). Poly(methyl methacrylate) and poly(ethylenimine) are still undergoing testing but it is hoped that this work will be published within a year.
Figure 5: PAA and PAH particle sizes when formulated at different solution pH values