Reports: ND155652-ND1: Conversion of Alkynes to alpha-Branched Products via Propargylic Substitution
Jeremy A. May, PhD, University of Houston
Summary of proposed goals in the grant application:
Aim 1: Synthesize a library of Tp ligands with variations in sterics and electronics and then use them to synthesize an array of organometallic complexes based on preliminary results with privileged substrates.
Aim 2A: Synthesize key inter- and intramolecular substrates to test if the complexes replicate or exceed current catalysts
Aim 2B: Test the new complexes against the array of substrates.
Results for Aim 1:
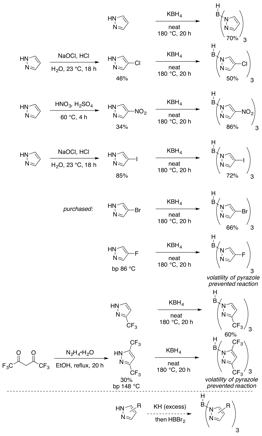
This aim has been the primary focus during this first year of the grant period. We have synthesized a small library of ligands that range in electronics from neutral to quite electron-deficient (Figure 1). Ligands with a larger steric profile were also synthesized. Nearly all of the desired ligands were obtained in useful yields. However, two exceptions were the fluorine-containing ligands, where the volatility of the pyrazole foiled the reaction since their boiling points were significantly less than the reaction temperature. To overcome this problem, we are developing a new trispyrazolylborate synthesis using the potassium salt of the pyrazoles and BHBr2.
Figure 1: Ligand Synthesis
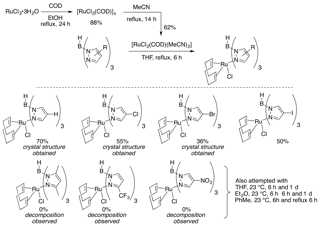
We have also identified two efficient strategies for synthesizing the Ru(II)Tp complexes from the relatively cheap precursor RuCl3. The first route reduces the ruthenium in the presence of COD and then forms the bisacetonitrile complex, which is primed for substitution (Figure 2). Exposure of this complex to the Tp ligand in refluxing THF provided the ligated metal for the halogenated Tp ligands. For the more sterically congested complexes, however, decomposition was observed. Thus, a second approach for metal ligation has been recently developed.
Figure 2: Ligand Addition to Ruthenium Strategy 1
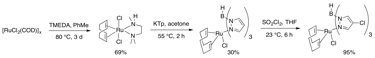
In the new strategy for metal ligation, RuCl2(COD) oligomer is treated with TMEDA to activate it for substitution (Figure 3). This may be directly substituted with the modified Tp ligand, or unsubstituted Tp ligand may be treated with an appropriate electrophile to modify it while ligated to the catalyst complex. This strategy is now being applied to the synthesis of the problematic substrates in Figure 2.
Figure 3: Ligand Addition to Ruthenium Strategy 2
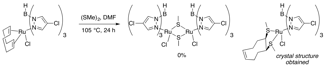
While we have had a promising result with TpRu(COD)Cl complexes, dimeric complexes have been the gold standard for propargylation reactions. In attempts to oxidatively form Tp analogs of these complexes, however, oxidation of the COD ligand occurs instead (Figure 4). Our strategy to circumvent this outcome will be to hydrogenate the COD ligand prior to reaction with MeSSMe.
Figure 4: Attempted Dimer Formation
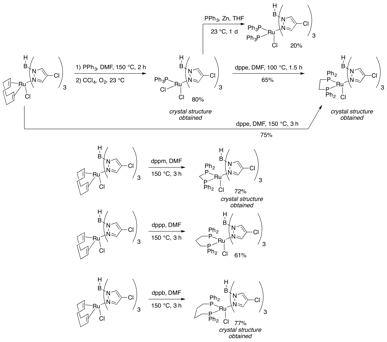
Based on indications that phosphine ligands may stabilize the 16-electron ruthenium active catalyst, we have also synthesized a number of phosphine-ligated ruthenium-Tp complexes (Figure 5). Both Ru(II) and Ru(III) complexes have been made, and monodentate or bidentate ligands incorporated.
Figure 5: Addition of Phosphines to the Complex
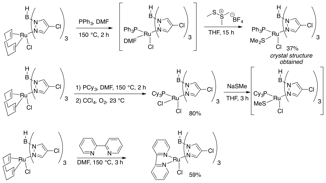
To further explore the substitution chemistry of these Tp complexes, the experiments in Figure 6 were made. In the first, a sulfur-ligated Me2S complex was isolated and characterized in another attempt to access a dimeric complex like that shown in Figure 4. We more closely approached the dimeric structure with the addition of NaSMe to a Ru(III) PCy3 complex. While the MeS adduct was observed in situ, loss of the phosphine did not occur to allow dimer formation. Attempts to isolate this intermediate failed, but we believe this is a good proof of principle that shows that we are close to our goal. Lastly, a diamine ligand was added to see any changes in catalytic activity. A range of P,N and P,O ligands will also be incorporated for that reason.
Figure 6: Additional Non-Phosphine Ligands
Results for Aim 2A:
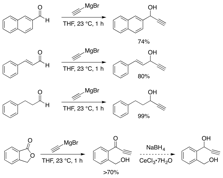
We have chosen to synthesize four simple, but representative, substrates to test the complexes above for catalytic activity. First is a naphthylated propargylic alcohol that is easy to monitor in the reaction and is a known privileged substrate for the propargylation of nucleophiles to serve as the positive control. A styrenyl alcohol has also been synthesized as a mechanistic indicator, as cation formation would lead to allylic mixtures of products, but a true allenylidene intermediate preserves the propargylic substitution. An aliphatic substrate has been synthesized for intermolecular reactions, and a somewhat privileged benzylic alcohol has been prepared to demonstrate intramolecular reactivity. Two aliphatic version of this last substrate are currently being synthesized: one for the formation of a tertiary ether, and the other for a quaternary ether as a model for quaternary carbon formation. A quaternary carbon model system will also be made from the propargylic ketone in Figure 7 via methyl-Li addition.
Figure 7: Substrate Synthesis
Results for Aim 2A: We have as yet made few trials of the complexes with the substrates in Figure 7.
Conclusion
The funding from the PRF has greatly accelerated our efforts in the synthesis of these catalysts. We are nearing the completion of the proposed libraries of ligands and ruthenium complexes and have developed new synthetic approaches in the process. The novelty of the complexes, most of which have been characterized via single crystal analysis, merits their publication independent of their catalytic activity. Substrate synthesis is also nearly complete, and catalytic testing is beginning and will continue in the second year of the grant period. We are highly optimistic in the potential for significant expansion in the substrate scope. The award has had a significant and beneficial impact on the PI's career in allowing him to explore a new field of research. Moreover, this in house library of catalysts is expected to support his synthetic efforts in a synergistic fashion and to allow for the discovery of new transformations that will contribute to his research program for years. Additionally, the graduate student performing this research has been financially supported in his pursuit of a PhD degree, which is necessary for his career goals as a scientific lead in the chemical industry. He has also benefited from exploring the intersection of inorganic synthesis with synthetic organic methods development, which would not have been possible without the award.