Reports: UR954875-UR9: Tailoring Ionic Liquids for Deep Desulfurization of Liquid Fuels by Oxidative Extraction
Hua Zhao, PhD, Savannah State University
During the first year of the ACS-PRF funded project, the following major tasks have been successfully accomplished:
1. We completed and published a comprehensive review on “Oxidative Desulfurization of Fuels Using Ionic Liquids (ILs)”. In this review, we systematically discuss the utility of ILs in catalytic extractive oxidation, including their roles as extractants, catalysts, or dual extracting/catalytic species for this application. We also point out the challenges of using ILs in this regard, including their relatively high costs and excessive viscosities, as well as the efficiencies and stabilities of catalysts presently being considered within them.
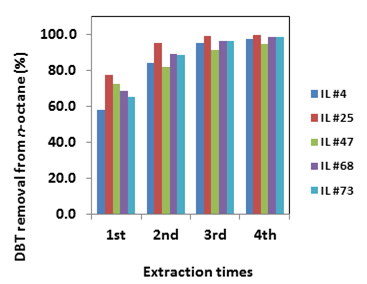
2. Due to a lack of systematic evaluation of the effect of IL structure on desulfurization efficiency, and a lack of mechanistic understanding regarding how ILs lead to the partition of aromatic sulfur compounds from fuel to the IL phase, we examined a total of 71 ILs and 2 deep eutectic solvents (DESs) with combinations of various cations and anions. We identified a number of ILs that produce high partition coefficients [up to 1.85 mg(S) kg (IL)–1/mg(S) kg (oil)–1] for the partition of aromatic sulfur compounds between ILs and n-octane or n-dodecane as surrogates for gasoline or diesel, respectively. We found that the high sulfur partition coefficient correlates with a high dipolarity/polarizability (π*) or a low solvent polarizability (SP) of ILs carrying the same cation and different anions, but correlates with a low dipolarity/polarizability (π*) for ILs carrying the same anion paired to cations bearing different alkyl chain lengths. The main partition mechanisms appear to be the hydrophobic interaction between cations and sulfur compounds, and the cation‒π interaction. In general, the combination of a more hydrophobic cation and a charge-dispersed anion results in a high partition of aromatic compounds into the IL phase. A more charge-dispersed anion often leads to a lower solvent polarizability, which enhances the cation–π interaction; on the other hand, a more hydrophobic cation results in a stronger hydrophobic interaction between ionic liquids and sulfur compounds. We further demonstrate that a four-step extraction using ILs (see Figure 1) could achieve 99% dibenzothiophene (DBT) removal (i.e., an initial sulfur content of 500 ppm is reduced to <5 ppm after the extraction).
Figure 1 Multi-step extractions of DBT (500 ppm) from n-octane by ILs.
3. One undergraduate student was supported by this project to participate in the research activities. The student received safety training and hands-on training on instruments (including HPLC, NMR and Karl Fisher titrator). The student assisted the PI in setting up the experiments, collecting and analyzing samples, and calculating data. The student is a co-author of one peer-reviewed paper along with the PI. The research experience made a huge impact on the student’s choices of career, and the student has been accepted into the Ph.D. program in Chemistry at the University of South Carolina beginning the Fall of 2016.