Reports: ND353842-ND3: Studies of Magnetic Properties of Metalloporphyrins by Inelastic Neutron Scattering
Ziling (Ben) Xue, PhD, University of Tennessee
Over the past year, we have focused our studies on the following: (1) Interpretations of inelastic neutron scattering (INS) data for manganese porphyrin complexes [Mn(TPP)X] (X = F, Br, I; H2TPP = meso-tetraphenylporphyrin); (2) Expansion of our INS studies to other metal complexes. INS experiments were performed at Oak Ridge National Laboratory (ORNL) and National Institute of Standards and Technology Center for Neutron Research. An article has been published in Inorganic Chemistry (IC), acknowledging PRF support. A second article, submitted to IC, is being revised based on reviewers’ comments. Both Ph.D. student Shelby E. Stavretis and Dr. Xue attended the 2016 American Conference on Neutron Scattering in July. Ms. Stavretis gave an invited talk at the conference. Dr. Xue also gave an invited talk at the 7th Chinese Chemical Society Physical Inorganic Chemistry Conference in May 2016. One Ph.D. student has been supported by the grant. Dr. Seth C. Hunter was supported before he recently accepted a position in industry.
Porphyrins in fossils, also known as geoporphyins, are the most abundant source of porphyrins on the earth. Geochemistry of metal complexes in fossils has been shown to be predominantly that of metalloporphyrins. Metalloporphyrins are often paramagnetic. We have probed metalloporphyrins by INS to obtain their zero-field splitting (ZFS) parameters (D), constants for compounds with S > 1/2. ZFS leads to magnetic anisotropy with profound effects on magnetic properties of the compounds. INS is a method to directly give the D values. To our knowledge, INS has not been used to study metalloporphyrins.
We have focused on [M(TPP)X] and [M(TPP)]. These are model metalloporphyrins with different transition metals, metals at different oxidation states, and compounds with different axial ligands.
I. INS Studies of [Mn(TPP)X]
We reported in 2014 and 2015 papers in IC ZFS parameters D of [Fe(TPP)X] (X = F, Cl, Br, I), [Mn(TPP)Cl], [Cr(TPP)Cl], and [Mn(TPP)]. The work on [Mn(TPP)X] (X = F, Br, I; Scheme 1) was designed to study halide effects on magnetic properties.
[Mn(TPP)F]. We have found that it is diamagnetic (S = 0). There are no ZFS (magnetic) excitations in its INS data. Magnetic susceptibility studies by Dr. Haidong Zhou in our physics department confirmed that [Mn(TPP)F] is diamagnetic. In contrast, halide analogs of [Mn(TPP)F] have four unpaired electrons (S = 2). For [Mn(TPP)Cl], D = -2.235(15) cm-1.
We hypothesize that [Mn(TPP)F] has a structure different from that of [Mn(TPP)Cl], perhaps with F- as bridging ligands between two Mn(III) ions. Through the bridging F-ligands, two neighboring Mn(III) ions anti-ferromagnetically coupled with no unpaired electrons. Since the crystal structure of [Mn(TPP)F] has not been reported, we have been working to grow its crystals. We will obtain the crystal structure of [Mn(TPP)F] to confirm our hypothesis.
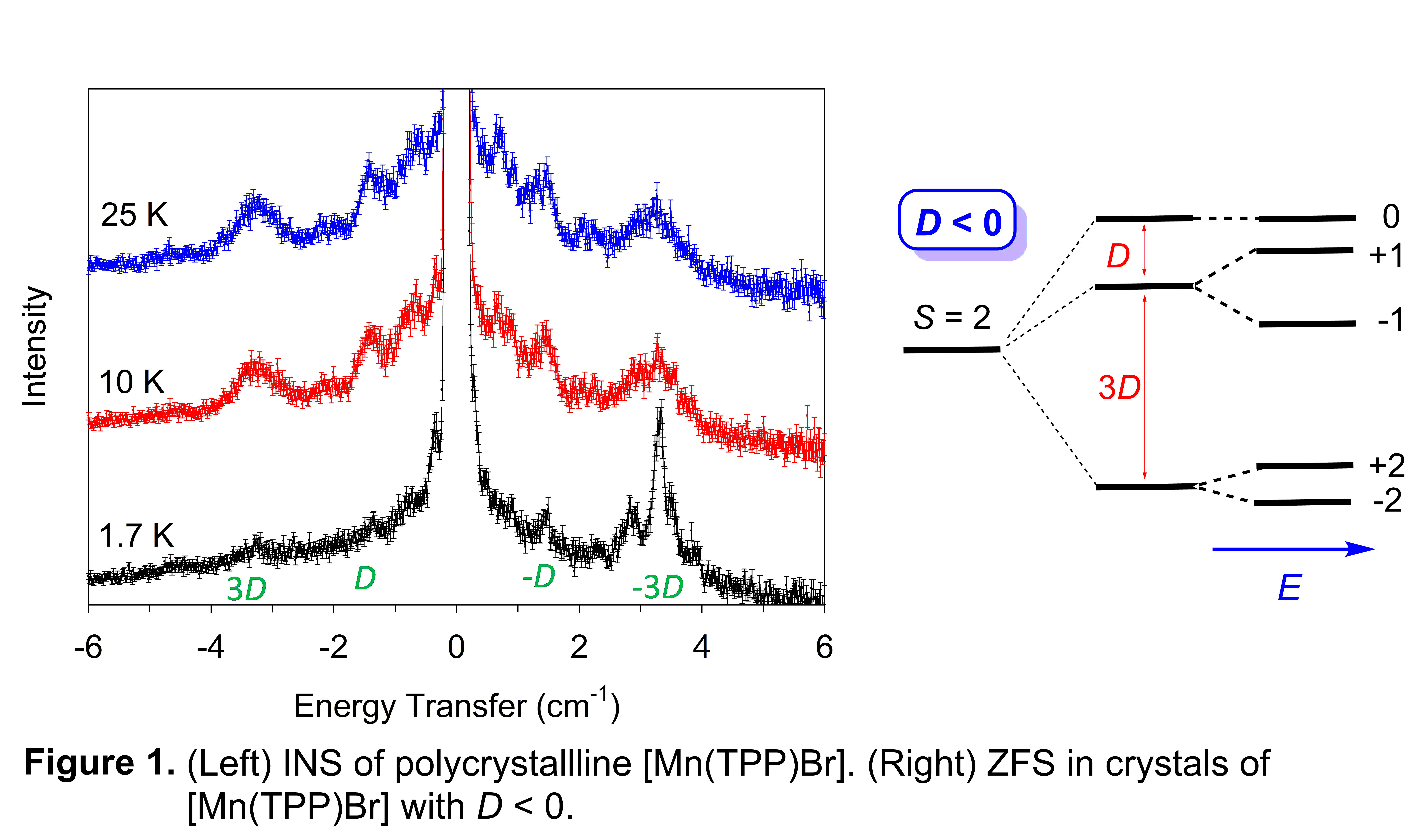
[Mn(TPP)Br] and [Mn(TPP)I]. Their INS spectra are given in Figures 1-Left and 2-Left, respectively. Although molecules of both complexes have 4-fold symmetry, their crystal structures do not have 4-fold symmetry. Thus their ZFS, shown in Figures 1-Right and 2-Right, are more complicated than those in Scheme 1-Right with the addition of the traverse anisotropy (parameter E). With E, fittings of the INS spectra to obtain both D and E are challenging.
[Mn(TPP)Br] and [Mn(TPP)I] have opposite signs of the axial ZFS parameter D: D < 0 for [Mn(TPP)Br] like Mn(TPP)Cl; D > 0 [Mn(TPP)I].
For [Mn(TPP)Br], D < 0 is shown by the temperature dependence in Figure 1-Left. The peaks around -3.3 cm-1 are present at lower temperatures while the peaks at -1.1 cm-1 become stronger at 25 K. Our initial estimate gave D = -1.07 cm-1 (if E = 0).
Attempts are underway to find the best fit of the INS data for D and E with the MAGPACK program. We have also studied [Mn(TPP)Br] by high-field electron paramagnetic resonance (HFEPR) through collaboration with Dr. Jurek Krzystek (National High Magnetic Field Laboratory, MagLab) and Dr. Joshua Telser (Roosevelt University) to confirm the INS results and help D and E calculations from INS spectra. HFEPR results gave D = -1.10 and E = 0.095 cm-1.
D > 0 for [Mn(TPP)I] is based on the temperature dependence in Figure 2-Left. The peaks around -0.9 cm-1 are present at low temperatures while the peaks around -2.7 cm-1 become stronger at 25 K. Initial estimate gave D = 0.90 cm-1 (if E = 0).
We are finding the best fit of the INS data for D and E with the MAGPACK program. We have also studied [Mn(TPP)I] by HFEPR which confirmed D > 0. Preliminary calculations gave D = 1.35 and E = 0.02 cm-1.
Axial ZFS parameters D in [Mn(TPP)X] are summarized in Table 1.
Table 1. Axial ZFS parameters D in [Mn(TPP)X]
| [Mn(TPP)F] | [Mn(TPP)Cl] | [Mn(TPP)Br] | [Mn(TPP)I] |
Axial ZFS D (cm-1)
| None (diamagnetic)
| -2.235(15)
| -1.10 (preliminary)
| +0.90 (preliminary)
|
II. INS Studies of Single-molecular Magnets (SMMs)
These studies are part of our attempts to expand our INS studies by this New Directions grant and compare effects of replacing porphyrins by other ligands. Co(acac)2(H2O)2 (acac = acetylacetonate), e.g., was reported by S. Gomez-Coca and coworkers (Nature Comm, 2014, 5, 4300) to behave as an SMM. In comparison, Co(TPP) (S = ½) does not have ZFS and Co(TPP)Cl is diamagnetic. ZFS in Co(acac)2(H2O)2 was found to be 2D » 114 cm-1. SMMs have potential applications as new data storage materials and in quantum computing.
We have studied INS of both Co(acac)2(D2O)2 and fully deuterated Co(acac-d7)2(D2O)2 (S = 3/2). Co(acac-d7)2(D2O)2 gives better signal/noise ratios. The sign of ZFS was investigated by seeing if the -1/2 ® +1/2 peak is revealed at 1.7 K with increasing magnetic field from 2 to 10 T in Figure 3. Indeed this peak appeared beginning at 2 T, confirming D > 0 for Co(acac-d7)2(D2O)2.
We were able to utilize the decreasing dependence of the scattering vector |Q| for the 113 cm-1 peak to reveal ZFS in Figure 4. Magnetic fields were applied to see how the ZFS peak changes. At 10 T, this peak intensity disperses, consistent with a magnetic peak.
We have studied other SMMs by INS and are processing data.