Reports: ND354167-ND3: Metals at Tripodal Sites of Adamantane and Graphane-Like Surfaces of Nano-Diamonds: A Unified Strategy for Petroleum-Derived Materials from the Molecular to the Nano-Scale
Ulrich Fekl, University of Toronto
We are synthesizing 2-adamantyl metal complexes with the goal to CH-activate and functionalize adjacent CH2 positions. The synthesis of 2-adamantyl anions is poorly developed, and we are currently adding to the synthetic toolkit available. While organometallic adamantyl anion equivalents are known, most reactions are either low-yielding or hard to reproduce to the extent that synthetic use has been limited.[1] For example, in the synthesis of adamantyl Grignards from adamantyl halides and magnesium turnings, the speed of stirring seems to drastically impact yield.[1f] Products are typically contaminated: using magnesium turnings or active Rieke magnesium[2] on bromoadamantanes produces significant amounts of homocoupled biadamantyls.[3] Further, most of the synthetic work on adamantyl anions focuses on the 1-adamantyl anion, with much less work on 2-adamantyl. A recent report by Knochel et al. describing the synthesis of 1-adamantylzinc halides was a major step toward reproducible and high yielding methods for the preparation of adamantyl anion equivalents.[4] In our PRF-funded work, we have significantly enlarged the number of adamantyl metal complexes known. Specifically, we have developed syntheses for several previously unknown 2-adamantyl metal complexes.
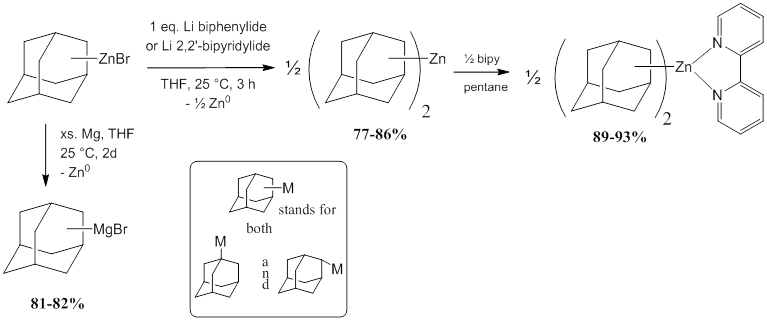
We discovered a good synthesis of the very carbanionic adamantylmagnesium bromides (both 1-AdMgBr and 2-AdMgBr): the reduction of the corresponding adamantylzinc bromides (accessible using Rieke zinc and also now commercially available) with excess magnesium turnings. The desired Grignards are produced cleanly (Figure 1, left).
Figure 1. New routes to 1- and 2-adamantyl anion equivalents.
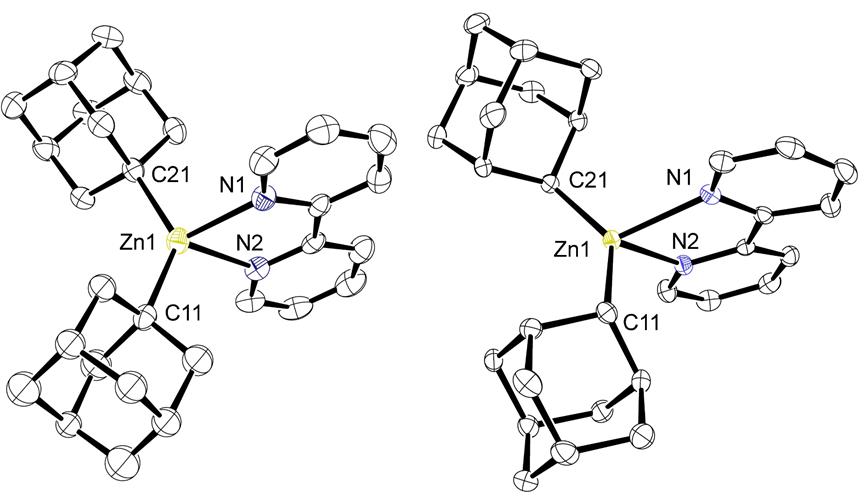
Using somewhat similar reductive techniques, reactions of adamantylzinc bromides with THF-solvated lithium biphenylide afford the new diadamantylzincs, namely 1-Ad2Zn and 2-Ad2Zn, by removing ZnBr2 from the equilibrium via reduction to zinc metal (Figure 1, right). In this case, exactly 1 equivalent of Li biphenylide is required. The addition of excess Li biphenylide results in formation of adamantane, most likely through the formation of adamantyl lithium compounds, which, however, rapidly deprotonate THF. We also found that use of lithium 2,2’- bipyridylide (Figure 1, right) offers an alternative approach to synthesis of 1-Ad2Zn and 2-Ad2Zn which does not require sublimation of the biphenyl byproduct. This method does not afford the 1-Ad2Zn(bipy) and 2-Ad2Zn(bipy) products but the homoleptic N-ligand free zinc diadamantyl species due to coordination of the bipyridine to lithium. The new routes produce ca. 80 % yield and offer significant advantages over existing routes due to their high degree of reproducibility even when performed using non-activated magnesium turnings (for the Grignards) or lithium chunks without the addition of sodium as an activator (for Ad2Zn synthesis). The new diadamantylzinc compounds can easily be isolated in pure solvent-free and salt-free form and are conveniently soluble in hydrocarbon solvents. Addition of 2,2’-bipyridine to 1-Ad2Zn or 2-Ad2Zn cleanly affords the crystalline complexes 1-Ad2Zn(bipy) and 2-Ad2Zn(bipy). Figure 2 shows the molecular structures of 1-Ad2Zn(bipy) and 2-Ad2Zn(bipy) from single crystal X-ray crystallography.
Figure 2. Structures of 1-Ad2Zn(bipy) (left) and 2-Ad2Zn(bipy) (right).
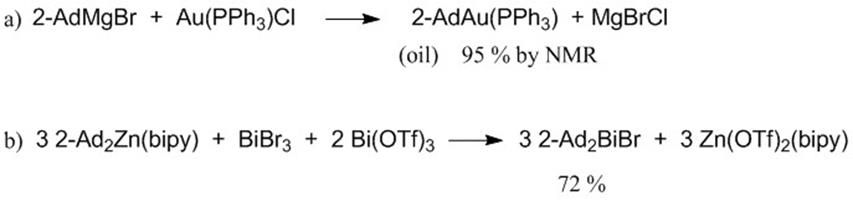
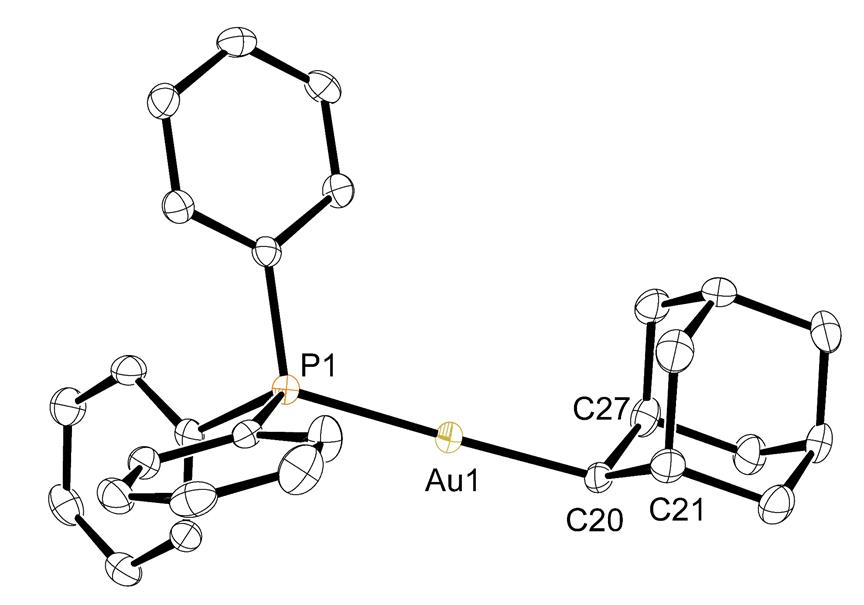
We have successfully transmetallated the 2-adamantyl anion onto gold and bismuth (Figure 3, a and b). The compounds were characterized by NMR spectroscopy and also X-ray crystallography (Figures 4 and 5). Bis(2-adamantyl)mercury was also synthesized and characterized (NMR and elemental analysis), although crystals have not been obtained yet for the mercury compound.
Figure 3. Transmetallations onto gold and bismuth.
Figure 4. Structure of 2-AdAu(PPh3)
Figure 5. Structure of 2-Ad2BiBr.
In conclusion, with PRF support we have developed new and high-yielding methods to make adamantyl metal complexes, in particular those of the hard-to-synthesize 2-adamantyl group. Future work will concentrate on intramolecular CH bond activation within these and related metal 2-adamantyls, in order to achieve novel functionalizations on the adamantane framework.
[1] (a) Wieringa, J. H.; Wynberg, H.; Strating, J. Syn. Comm. 1971, 1, 7-10. (b) Wieringa, J. H.; Strating, J.; Wynberg, H. Syn. Comm. 1972, 2, 191-195. (c) Wieringa, J. H.; Wynberg, H.; Strating, J. Tet. Lett. 1972, 20, 2071-2074. (d) Molle, G.; Dubois, J. E.; Bauer, P. Tet. Lett. 1978, 34, 3177-3180. (e) Molle, G.; Dubois, J. E.; Bauer, P.; Syn. Comm. 1978, 8, 39-43. (f) Molle, G.; Bauer, P.; Dubois, J. E. J. Org. Chem. 1983, 48, 2975-2981. (g) Molle, G.; Briand, S.; Bauer, P. Dubois, J. E. Tetrahedron, 1984, 40, 5113-5119.
[2] Rieke, R. D.; Bales, S. E. J. Am. Chem. Soc. 1974, 96, 1775-1781.
[3] Molle, G.; Bauer, P.; Dubois, J. E. J. Org. Chem. 1982, 47, 4120-4128.
[4] Sämann, C.; Dhayalan, V.; Schreiner, P. R.; Knochel, P. Org. Lett. 2014, 16, 2418-2421.