Reports: DNI354907-DNI3: Two-Stage Catalytic Aerobic Oxidation of Alkanes with Iridium Pincer Complexes
Thomas S. Teets, University of Houston
Alkanes are a major fraction of naturally occurring petroleum resources, and are also significant products in catalytic cracking and Fischer-Tropsch synthesis. Whereas certain alkane fractions, particularly those with Cn>4 are valuable as transportation fuels (gasoline, diesel, and jet fuel), lighter fractions are frequently burned during the extraction or refining processes. Chemical transformations which could selectively upgrade these alkane fractions into value-added products would be both economically and environmentally beneficial, allowing resources to be used more efficiently and reducing waste. Pincer iridium complexes are among the best-known homogeneous catalysts for the activation and functionalization of alkanes, particularly for alkane dehydrogenation to alkenes,1 with most the well-known catalysts utilizing electron-rich, air-sensitive supporting ligands and requiring intermediacy of a reactive 14-electron Ir(I) species. Armed with recent precedents that iridium(III) complexes with aerobically stable pincer ligands can promote stoichiometric alkane dehydrogenation in the presence of oxygen,2–4 this work seeks to develop next-generation iridium(III) catalysts which can promote aerobic functionalization of alkanes. Specifically, a two-step catalytic reaction is envisioned where alkane dehydrogenation is followed by aerobic functionalization of the alkene to a carbonyl compound, with both steps using the same catalyst.
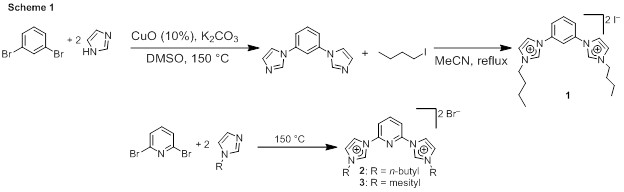
This work has targeted precatalysts with N-heterocyclic carbene (NHC) pincer ligands, which are strongly coordinating, oxidatively robust, thermally stable, and easy to synthetically modify. Much of the early work on this project has involved the synthesis of NHC pro-ligands and their iridium complexes. The student on this project, Kusun Choung, had little experience in transition metal chemistry prior to joining this new project, and the support of the ACS PRF has enabled his training in a new area of chemistry. Scheme 1 shows the synthesis of the imidazolium precursors for the anionic “CCC” (1) and neutral “CNC” (2 and 3) pincer ligands this work has targeted. The CCC precursor is prepared in a two-step process where copper-catalyzed aryl amination to prepare the bis-imidazole is followed by alkylation with butyl iodide. The CNC precursors are prepared via a slightly different route, with uncatalyzed nucleophilic aromatic substitution between 2,6-dibromopyridine and the alkyl or aryl imidazole producing the desired product.
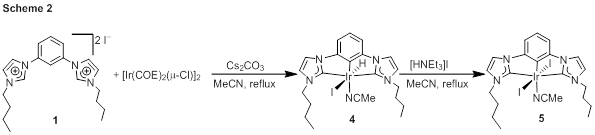
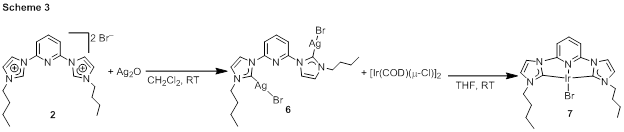
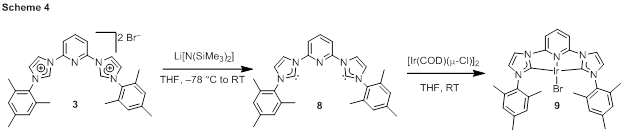
Iridium complexes of 1–3 were prepared in three ways. In one method, summarized in Scheme 2, the imidazolium precursor 1 was treated with [Ir(COE)2(μ-Cl)]2 (COE = cyclooctene) in the presence of cesium carbonate base. Three base-promoted C–H addition reactions produce the iridium(III)-CCC hydride complex 4, which has been characterized by NMR spectroscopy. Treatment of this complex with triethylammonium iodide furnishes the iodide complex 5. CNC complexes were not accessible by this route, so instead two additional strategies have been found to be viable for accessing complexes of 2 and 3. For alkyl-substituted ligand precursor 2, the free carbene is not expected to be stable, so a transmetalation reaction was used to avoid intermediacy of the free NHC (Scheme 3). First silver complex 6 was prepared by reaction of 2 with Ag2O, and then this intermediate was treated with [Ir(COD)(μ-Cl)]2 (COD = 1,5-cyclooctadiene) to furnish 7. For mesityl-substituted imidazolium 3, it was possible to first deprotonate with strong base to yield free carbene ligand 8, which was then treated directly with [Ir(COD)(μ-Cl)]2 to produce 9. Both 7 and 9 are iridium(I) complexes, and the proposed catalytic reactions involve air-stable iridium(III) pre-catalysts, so efforts are underway to chemically oxidize 7 and 9 to corresponding iridium(III) complexes.
With complexes 4, 5, 7, and 9 in hand, the PI’s group has begun to explore catalytic reactions and stoichiometric reactions relevant to elementary steps in the proposed catalytic cycle. Preliminary results have shown that complex 5 is active for alkane dehydrogenation to alkenes, although thus far catalytic activity has not been observed. The complexes described in Schemes 2–4 also permit the study of reactions that model elementary steps in the proposed catalytic cycle, and these studies are also currently being carried out. In particular, hydroxo complexes analogous to halide complexes 5, 7, and 9 are being sought. C–H activation by a terminal hydroxo complex is an important elementary step in aerobic dehydrogenation schemes. In addition, the reactivity of hydride complex 4 towards O2 is an important elementary step which is being studied currently in the PI’s group.
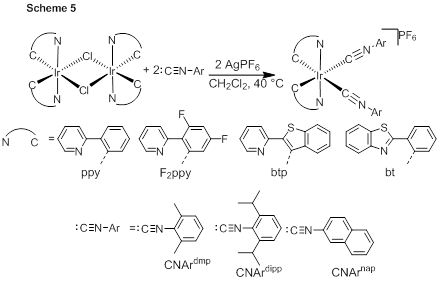
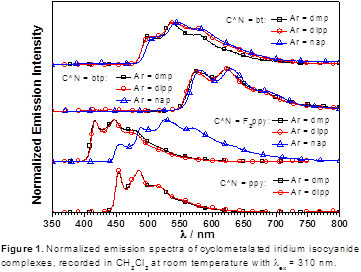
Support from the ACS PRF has enabled the preliminary work on the project described above, which will require much additional time investment before coming to full fruition. However, the ACS PRF grant has also partially supported a postdoctoral associate, Ayan Maity, and allowed the PI to explore his other interests in molecular phosphors. The work on this second project, summarized briefly in this report, also involves coordination chemistry of iridium, including carbene-based ligands, but with the goal of developing cyclometalated iridium(III) complexes with enhanced optical properties. Scheme 5 shows the synthesis of a series of cyclometalated iridium(III) complexes with ancillary aryl isocyanide ligands. Initial work on these complexes has demonstrated that they are efficient, color-tunable phosphors, with emission spectra shown in Figure 1. In unpublished work that began more recently, the PI’s group has begun investigating the reactivity of the complexes described in Scheme 5 and related variants. Reaction with amine or hydrazine-based nucleophiles converts the isocyanide ancillary ligands into acyclic carbene or chelating dicarbene ancillary ligands, structures which are not accessible by traditional synthetic approaches and may lead to the discovery of complexes with enhanced photophysical properties. Thus, the PRF support has allowed the PI to pursue the primary project goals of alkane dehydrogenation using iridium(III) complexes while simultaneously making possible efforts in iridium(III) coordination chemistry related to the development of molecular phosphors.
References
(1) Choi, J.; MacArthur, A. H. R.; Brookhart, M.; Goldman, A. S. Chem. Rev. 2011, 111, 1761–1779.
(2) Ito, J.; Kaneda, T.; Nishiyama, H. Organometallics 2012, 31, 4442–4449.
(3) Allen, K. E.; Heinekey, D. M.; Goldman, A. S.; Goldberg, K. I. Organometallics 2013, 32, 1579–1582.
(4) Allen, K. E.; Heinekey, D. M.; Goldman, A. S.; Goldberg, K. I. Organometallics 2014, 33, 1337–1340.