Reports: UNI353723-UNI3: Chelation-Assisted Au(III) Functionalization of Strong, sp3-Hybridized C-H Bonds, Similar to Those Found in Alkanes
David R. Weinberg, PHD, Colorado Mesa University
This grant has allowed my research group to isolate and characterize novel gold(III) complexes for the purpose of developing gold(III) C-H bond functionalization catalysts. It has also allowed me to train seventeen undergraduate researchers and to help educate local primary school age children regarding the utilization of petroleum and other energy sources.
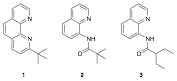
My research focuses on developing gold(III) catalysts for inner-sphere, homogeneous functionalization of strong, sp3-hybridized C-H bonds, similar to the bonds found in alkanes. Through this grant, we have developed conditions for the syntheses of novel gold(III) complexes. These complexes contain ligands designed to facilitate the activation of strong, sp3-hybridized C-H bonds by directing these bonds toward the gold(III) metal center through chelation. We have developed syntheses of complexes containing the ligands (1, 2, and 3) shown in Fig. 1.
Figure 1. Ligands 1, 2, and 3.
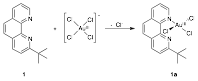
Ligand 1 can be metallated with tetrachloroaurate(III) to generate a monomeric gold(III) complex (1a) as indicated in Fig. 2 using two different sets of conditions. While no reaction occurs between 1 and tetrachloroaurate(III) at room temperature in most organic solvents, 1a is generated in acetonitrile when AgBF4 is included in the reaction mixture for the purpose of abstracting a chloride from gold(III). The resulting gold(III) complex can also be formed in the absence of AgBF4 when the reaction is performed in a mixture of an organic solvent (THF or acetonitrile) and water. Presumably, the water helps to labilize the chlorides bound to gold(III). Surprisingly, the yield and purity of 1a increased in the organic solvent/water mixtures when one equivalent of sodium bicarbonate was added to the reaction mixture. While metallation to form 1a probably does not involve a proton transfer, it is possible that the sodium bicarbonate is preventing alternative reaction pathways or that sodium bicarbonate is affecting ligand substitution at the gold(III) metal center.
Figure 2. The synthesis of 1a. This occurs in CH3CN with AgBF4 or in a mixture of an organic solvent (THF or CH3CN) with H2O.
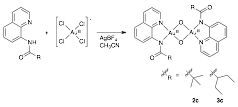
The conditions used for the synthesis of 1a were also utilized in the metallation of 2 and 3. While the metallation of 1 led to a monomeric complex (1a) regardless of which set of conditions was used, 2 and 3 reacted in acetonitrile with tetrachloroaurate(III) in the presence of AgBF4 to generate the oxo-bridged gold(III) dimers 2c and 3c, respectively, as shown in Fig. 3. The oxo-groups likely arise from adventitious water present in the acetonitrile. Evidence for these dimers was obtained by NMR spectroscopy and elemental analysis; we are currently attempting to obtain crystals of these complexes for single crystal X-ray diffraction studies.
Figure 3. The generation of oxo-bridged gold(III) dimers 2c and 3c.
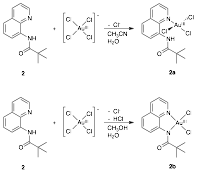
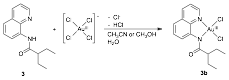
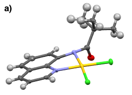
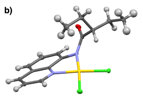
Monomeric gold(III) complexes can be generated with ligands 2 and 3 when the metallations are performed in the absence of silver(I) salts and instead in mixtures of organic solvents with water. These metallations are shown in Figs. 4 and 5. While solvent mixtures of both acetonitrile and methanol with water resulted in bidentate binding of 3 to gold(III), 2 bound to gold(III) in a bidentate fashion when metallated in a mixture of methanol and water, but monodentate binding of 2 to gold(III) resulted from a mixture of acetonitrile with water. Metal binding of the amide in 2 is likely sterically hindered by the proximity of the quaternary carbon. In comparing the crystal structures of 2b and 3b in Fig. 6, it can be seen that the different alkyl groups in these complexes impose significantly different amide geometries. Furthermore, the differences in the geometries seem to have significant effects on the electronic structures of the complexes, causing 2b to be green, while 3bis yellow.
Figure 4. The metallation of ligand 2 in mixtures of organic solvents with water to generate the monomeric gold(III) complexes 2a and 2b.
Figure 5. The metallation of ligand 3 in a mixture of an organic solvent with water to generate the monomeric gold(III) complex 3b.
Figure 6. The single crystal X-ray diffraction structures of a) 2b and b) 3b.
The research students supported by this grant gained experience in ligand syntheses, metal complex syntheses, and air-free chemical techniques, including the use of both a Schlenk line and an inert atmosphere glove box. The glove box was purchased as capital equipment through this PRF grant with matching funds provided by Colorado Mesa University.
The research and glove box funded through this grant have also been used to improve my capstone Advanced Laboratory course. One of the projects in this course involves synthesizing some of the ligands described above. This gives the students experience using the Schlenk line, column chromatography, and NMR spectroscopy. I have the students read the PRF grant proposal in order to introduce them to the project and to the format of grant proposals. I have also incorporated training on the glove box into this course.
The funding of this project has had an impact on the broader Grand Junction community. My research group and I have given four presentations on energy to local middle school and elementary school students. These presentations were performed in collaboration with the John McConnell Math & Science Center, the Central Library of Grand Junction, and a Colorado Mesa University STEM camp for middle school students. For each presentation, we utilized a variety of demonstrations, and we taught the students about energy sources and about transfers of energy involved in chemical reactions. I also met with the Operations Manager of the John McConnell Math & Science Center to discuss how they could update their displays related to the utilization of the world's energy resources.
My research group and I are exceptionally grateful for this grant. It has allowed us to develop syntheses of novel gold(III) complexes, and seventeen undergraduate research students have been trained in a variety of synthetic inorganic chemistry techniques. Furthermore, it has helped me to improve my capstone Advanced Laboratory course, and it has helped to facilitate my research group's involvement in the education of local children.