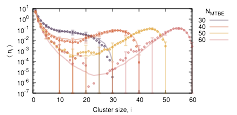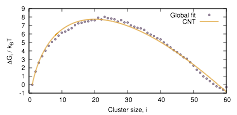Reports: ND654642-ND6: Molecular Simulation Studies of Clathrate Hydrate Phase Nucleation
James T. Kindt, Emory University
Summary: The project goal was to study the formation of methane clathrate hydrate using MD simulation coupled with recently developed analysis tools. These "small-N" cluster analysis tools yield cluster free energies from equilibrium cluster size distributions, even in cases where relatively few clusters are present and the law of mass action does not apply. Taking the advice of the proposal's reviewers, we have pursued some less complex systems to refine our methods before tackling the original problem.
Nucleation of liquid MTBE from vapor and solution phases: Methyl t-butyl ether (MTBE) is a fuel additive whose moderate solubility in water has led to issues environmental contamination. To explore capabilities and limitations of the small-N methods, MTBE cluster formation in vapor and solution environments have been studied. PhD student Lara Patel has developed a global fitting procedure to incorporate results from simulations that cover overlapping distributions of cluster sizes and find the optimal set of cluster free energies for the entire data set. She has applied this procedure to simulations of MTBE cluster formation from the vapor and from aqueous solution. The shape of the cluster free energy curve is fit well using a function derived from classical nucleation theory but with a somewhat lower apparent surface tension for the clusters than for planar interfaces. A report of this work has been submitted to the Journal of Chemical Theory and Computation.
Figure 1a: Simulated MTBE cluster size distributions data (symbols) and predictions of best-fit set of cluster association free energies ÆG (curves) for MTBE in aqueous solution.
Figure 1b: Globally constrained cluster free energies (circles) and best-fit approximation using classical nucleation theory expression (curve).
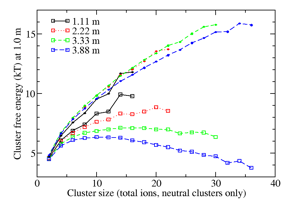
Formation of amorphous NaCl clusters from aqueous solution: Evidence from recent simulation studies by Chakraborty and Patey1 suggests that the formation of a dense amorphous NaCl phase precedes formation of ordered crystallites. Lara Patel has investigated the formation of the amorphous clusters themselves. This has required the development of theory to treat two-component systems. In an initial test application to small clusters of Na+ and Cl- at moderate concentration, we found strong concentration dependences in the best-fit cluster free energies (Figure 2, open squares) implying that the equilibrium constants are not constant. Hypothesizing that this arose from the crowding among ions and clusters (with their solvent shells), we adapted our analysis method to account for the excluded volume effects of clusters' solvent shells. After analyzing the solvation structure in simulations to find the volumes of solvation shells associated with s-mer clusters, the cluster analysis was repeated with the effective volume of the system reduced by half of the total volumes of the clusters' solvent shells; the factor of one-half reflects the ability of the same water to solvate two ions. In dilute systems, this had a negligible effect on the size distributions, but at high concentration it produced a shift towards large clusters, as water to solvate fully separated ions becomes scarce. Agreement between the recalculated cluster free energy curves at different concentrations in Figure 2 (filled circles) indicates success in disentangling intrinsic cluster stability from crowding effects.
Figure 2. Cluster free energies of neutral NaCl clusters from fitting of full binary cluster distributions for 20 NaCl in water, simulated over 500 ns at 300 K. Open squares are based on ideal cluster mixing with solvent; filled circles are corrected for crowding effects.
Expecting that crowding effects would disappear and cluster association constants would be well-behaved for a system with a lower saturation concentration, we have extended the investigation to NaCl in methanol solution. Here the behavior is quite different, and clusters as small as 12 ions begin to show rock-salt crystalline structure in dynamic equilibrium with other structures.
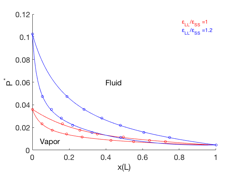
Vapor-liquid coexistence in size-asymmetric mixtures. A second student, Ziwei Guo, has been partially supported through this grant. He has adapted a recently developed Monte Carlo (MC) algorithm to perform grand canonical ensemble simulations of mixtures of Lennard-Jones particles in gas and liquid phases, determining the phase boundaries for mixtures with a 2:1 size discrepancy, greater than accessible through previous methods. The challenge here is in achieving insertion moves into a dense fluid where one large particle occupies a volume eight times that of a small particle; the challenge is met by a careful exchange of a single large particle for multiple small particles, which we call "solvent repacking" step. The same student has also analyzed results of grand canonical solvent repacking Monte Carlo simulations investigating the ordering of hard spheres at the surface of a much larger sphere, which is a model for "colloidosome" colloidal assemblies of potential interest as microencapsulants.
Figure 3: phase boundaries enclosing vapor/liquid coexistence regions, obtained from MC simulation of two systems with particle size ratio 2:1 and different particle energy ratios shown, in terms of reduced pressure P* and mole fraction of large particles, at temperature T=ε/kB.
Career impact on supported personnel: In addition to supporting the stipend of the Ph.D. students who performed this work (Lara Patel and Ziwei Guo) and paying for essential computational resources, this funding allowed Lara and the PI to attend the American Physical Society March Meeting and present research talks. One manuscript has been submitted, one more is in preparation, and two others are expected to result from these supported activities. The results obtained will also be included in two NSF proposals by the PI.