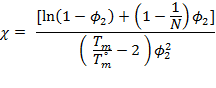Reports: DNI755456-DNI7: Miscibility and Structures of Polycyclic Aromatic Hydrocarbons and Polymeric Materials
Sangwoo Lee, Rensselaer Polytechnic Insittute
Polycyclic aromatic hydrocarbons (PAHs) are compounds made of fused benzene rings, and the conjugated π electrons in PAHs gives unique chemical, physical, and optical properties. PAHs are abundant in crude oils and other fossil fuels, key by-products in combustions, and even major hydrocarbon compounds in the extraterrestrial space. However, despite of the interesting properties and large material availability, PAHs cannot be easily incorporated with other materials because of their high immiscibility with other compounds due to the strong inter-PAH π-π interactions and planar geometry causing entropic penalty.
Among many unique properties of polymers compared to simple molecules, polymers can combine chemical functions of simple monomeric molecules in a spatially controlled manner by covalent bonding and these structural features make polymers have different physical states to these of monomeric molecules with conserving the chemical functions of monomers. Thus, polymerization enables decoupling physical properties from the chemical properties of monomer compounds for target functions, and has high potentials as solubilization matrix for PAHs if proper chemical functions are incorporated.
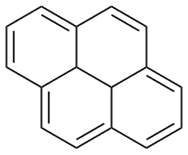
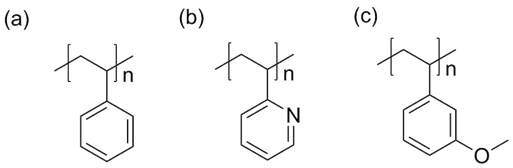
We investigated the interaction parameter between pyrene and polystyrene-derivative polymers to examine the solubility of PAHs in polymer matrix to gain insights on the thermodynamic properties. Pyrene is a simple PAH made of four fused benzene rings with a melting temperature T°m = 155 °C (Figure 1). This simple molecule has the unique properties of PAHs and its relatively low Tm makes the investigation of miscibility with polymers facile task. Figure 2 shows the molecular structures of polystyrene derivatives with their molecular weights utilized in this study so far: polystyrene, poly(2-vinylpyridine), and poly(3-vinylanisole). These polymers were all synthesized by anionic polymerization technique and all have mono-modal and narrow molecular weight distribution (Đ ≤ 1.1).
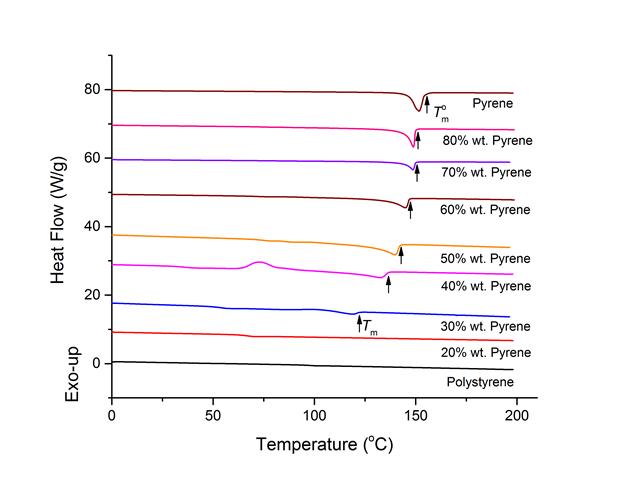
Pyrene blended with polymers form crystals at low temperatures, but form homogeneous liquid states at elevated temperatures. However, pyrene showed different melting temperatures by polymer species and concentrations, which suggests the interaction between pyrene and segments vary by polymer and concentration. We utilized the melting points of pyrene with polymer extracted from differential scanning calorimetry (DSC) technique for evaluation of the interaction parameter χ between pyrene and polymers by the following equation based on the classical Flory-Huggins regular solution theory:
where ϕ2 is the volume fraction of polymer, N is the degree of polymerization of polymers based on the unit volume of pyrene, and Tm and T°m are the melting points of pyrene with polymer and without polymer respectively. Figure 3 shows a representative DSC thermogram of pyrene and polystyrene blends. In the experimental determination of melting temperature of pyrene, the higher on-set points were chosen to account in the reduced melting temperature by size of pyrene crystal grains.
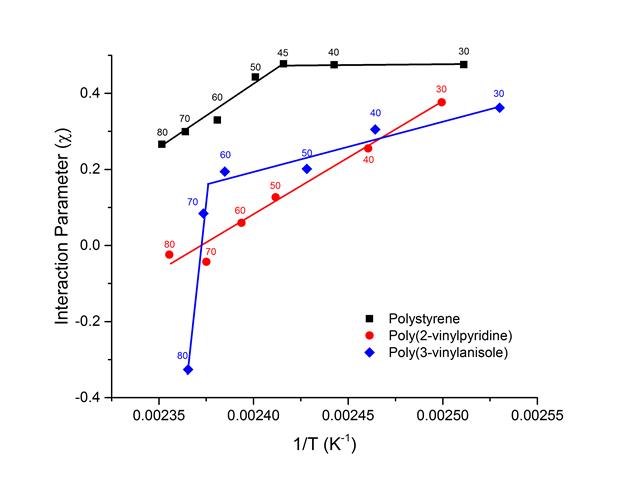
The extracted interaction parameters between pyrene and polymer compounds shown in Figure 4 displayed interesting trends, suggesting non-simple phase states in these binary blends. Polystyrene showed higher interaction parameter than the other two polymers, poly(2-vinylpyridine) and poly(3-vinylanisole), indicating the phenyl side groups along polyethylene main backbone chains have least compatibility with pyrene among the polymer species investigated. This is possibly due to that the other polymers have side groups with larger dipole moments inducing stronger van deer Waals interactions with pyrene. Interestingly, the interaction parameter with polystyrene and poly(3-vinylanisole) showed discontinuous changes in interaction parameters by composition, which suggest drastic changes in the spatial correlation between pyrene and polymer segments may occur. Although it is daunting to elucidate the changes suspected in the spatial correlations between pyrene and polymer chain segments, any qualitative changes between pyrene-polymer liquid solutions and pyrene crystals should be reflected by molecular configurations in both states. Indeed, we identified changes in pyrene crystal structures depending on the pyrene and polymer concentrations (not shown) and the crystal structures are currently being investigated.
The current research outcomes revealed that polymers have favorable compatibility between PAHs and this provides a new approach to control the phase states of PAH-containing inhomogeneous molecules such as asphaltenes. The overall theme of this project promotes the project participants, graduate student and PI, to develop deeper insights on the thermodynamic behaviors of mixtures of macromolecular materials.
Figure 1. Molecular structure of pyrene.
Figure 2. Polymers blended with pyrene. (a) polystyrene with molecular weight Mn = 23.4 kg/mol, (b) poly(2-vinylpyridine) with Mn = 22 kg/mol, and (c) poly(3-vinylanisole) with Mn = 18 kg/mol.
Figure 3. Differential scanning calorimetry thermograms of pyrene and polystyrene blends. The curves are from heating at 10 °C/min after cooling from 200 °C to – 40 °C at 10 °C/min.
Figure 4. Interaction parameters between pyrene and polymer. Lines are to guide eyes.