Reports: UR1053614-UR10: Supercritically functionalized nanostructures for internal methane reforming
Alevtina Smirnova, PhD, South Dakota School of Mines and Technology
Within the third year of reporting, two groups of ceramic materials relevant to the SOFC anodes, cathodes, and the SOFC electrolyte have been synthesized, characterized, and tested.
PART I: Samarium and praseodymium-based perovskites
Perovskites were extensively studied in regard to their catalytic properties in SOFC cathodes and as SOFC anodes [1] to improve the internal reforming capabilities. Their structure and the electron configuration, e.g. A- or B-site doping, are considered as the main factors that define the reaction kinetics due to formation of various intermediates and multiple reaction pathways that could be rate-limiting. The B-site transition metal ions in an ABO3-δ perovskite perform an important role in binding the oxygen intermediates on the surface of the perovskite particle and, thus, affect the overall catalytic and fuel reforming efficiency. This mechanism is based on the molecular orbital bonding framework formed as a result of the corresponding interactions between the e.g. transition metal orbitals with negatively charged oxygen adsorbates and the occupancy of the eg orbital.
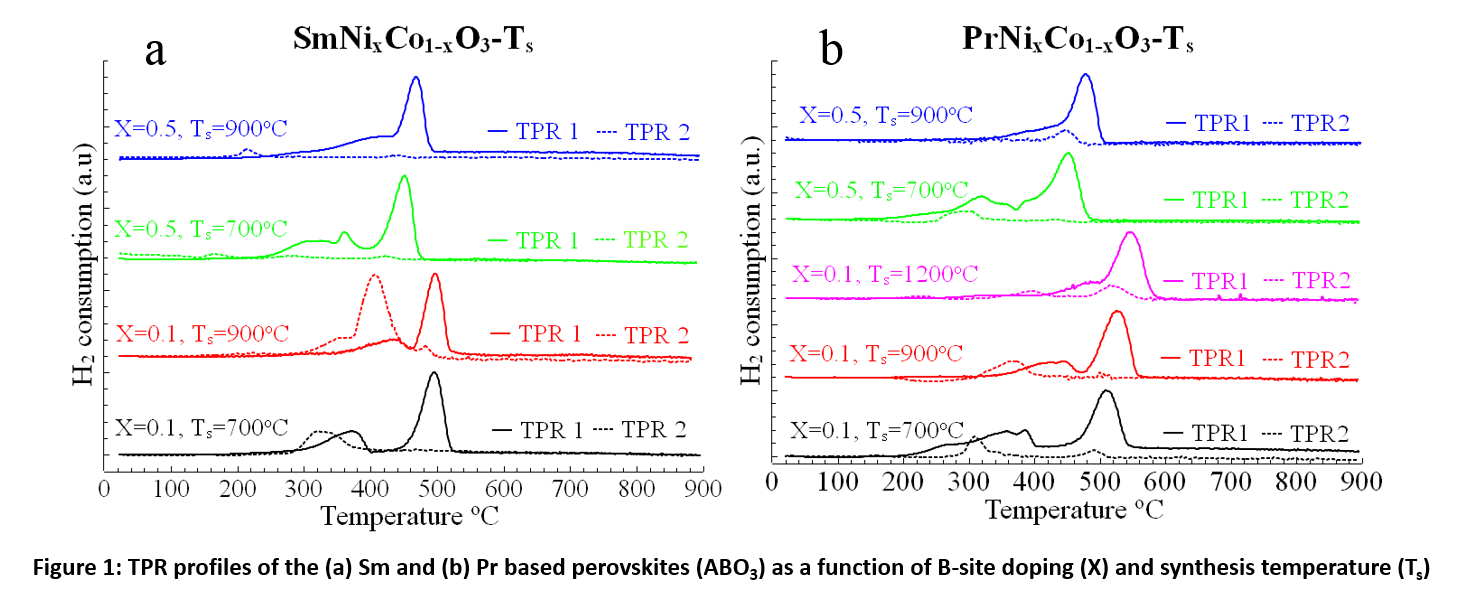
The goal of this study was to investigate the chemical stability of the doped SmNixCo1-xO3-δ and PrNixCo1-xO3-δ perovskites in the reducing environment relevant to the SOFC anodes. The effect of the B-site doping as a function of nickel to cobalt molar ratio (x= 0.1, 0.5, 0.9) vs. the heat-treatment temperature is presented in Figure 1 [2].
Figure 1: TPR profiles of the (a) Sm and (b) Pr-based perovskites (ABO3) as a function of B-site doping (X) and the synthesis temperature (Ts).
The redox behavior was studied using Temperature Program Reduction (TPR) by ramping the temperature from the room temperature to 900°C at 5°C/min in H2/Ar atmosphere, corresponding to the SOFC conditions on the anode. Two successive TPR cycles for each catalyst material were compared: TPR1 for measuring H2 consumption of as-synthesized perovskite, and TPR2 cycle after exposing the previously reduced material to ambient atmosphere for 24 hrs (Figure 1). The difference between the H2 consumption during TPR1 and TPR2 is a direct measure of change in the thermal stability and redox behavior of the perovskites. The TPR1 profiles of all the materials represents a typical perovskite reduction behavior by having two distinctive peaks. The first peak at lower temperatures (between 300-400°C) represents the formation of an oxygen-deficient perovskite structure while the second peak (> 400°C) indicates a complete reduction of perovskite structure. In the case of Sm-based perovskites, SmNi0.1Co0.9O3 synthesized at 900°C has shown best thermal stability and redox behavior. Whereas Pr-based perovskites show a significant differences in the reduction behavior as a function of their synthesis temperature and B-site doping [3].
Part II: Lanthanum molybdenum oxides (LAMOXs) as electrolyte materials for SOFC electrolytes
La2Mo2O9 (LAMOX)-based oxides are oxide-ion conductors that possess high oxide-ion conductivity at medium temperatures and, thus, have a potential to be used as solid oxide fuel cell electrolytes. Their high anionic conductivity is provided by a high concentration of intrinsic oxygen vacancies in the crystal lattice providing the oxygen ion diffusion. However, for parent La2Mo2O9, an abrupt increase of conductivity by almost two orders of magnitude was observed at about 580 °C due to a phase transition from a monoclinic low-temperature α phase to a cubic high-temperature β-form.
A series of doped La2-xRxMo2-yWyO9 (R=Bi, Gd, Pr, Sm, Nd; x=0–0.5, y=0.5–1) were synthesized by polymerized precursor method. The structural properties doped LAMOXs have been studied using thermal analysis, XRD, and TEM. The cubic β-phase formed from polymerized precursors or MM after calcination at 700–900 °C was stabilized at room temperature. Due to a high dispersion of powders, high density pellets of doped LAMOX ceramics have been obtained after sintering at 950°C. LAMOX doping lead to the higher electrical conductivity as compared with pure La2Mo2O9 in the temperature range of 400-570°C while above 570°C the conductivity was higher only in the case of W-doped LAMOX: 4.7-5.6 · 10-3 S/cm at 630°C [4].
[1] A. Kulkarni, F.T. Ciacchi, S. Giddey, C. Munnings, S.P.S. Badwal , J.A. Kimpton, D. Fini, Mixed ionic electronic conducting perovskite anode for direct carbon fuel cells, Int. J. Hydrogen Energy, 37(24) (2012) 19092–19102.
[2] P. Kolla, R. Rajappagowda, and A. Smirnova, The role of samarium-based perovskite-graphene nanocomposites for oxygen reduction and oxygen evolution in energy generation and storage (2016); Proceedings of the IEEE International Conference on Electro Information Technology (EIT).
[3] V. Sadykov, N. Eremeev, V. Bolotov, Y. Tanashov, Y. Fedorova, D. Amanbayeva, A. Bobin, E. Sadovskaya, V. Muzykantov, V. Pelipenko, and A. Smirnova, The effect of microwave sintering on stability and oxygen mobility of praseodymium nickelates-cobaltites and their nanocomposites, Solid State Ionics, 288 (2016) 76–81.
[4] S. Pavlova, Y. Bespalko, V. Sadykov, N. Eremeev, T. Krieger, E. Sadovskaya, A. Ishchenko, A. Bobin, A. Ulihin, N. Uvarov, and A. Smirnova, Structural and transport properties of doped LAMOX — Electrolytes for IT SOFC, Solid State Ionics, 288 (2016) 103–109.