Reports: DNI1052827-DNI10: Elucidating the Electronic Structure and Chemistry of Layered Vanadium Oxides for Next-Generation Energy Storage
Louis Piper, PhD, Binghamton University
Overview:
Our ACS PRF funded project has focused on the evolution of the electronic structure of promising materials for next generation Li-ion battery cathodes. Recently, nano-engineered LiFePO4 olivine has begun to replace costly and thermally unstable LiCoO2. The use of polyanions adversely reduces the specific capacity; one way to compensate for the loss of capacity is to develop a material containing a polyatomic anion (thermally stable) that is capable of inserting two lithium (or sodium) atoms per redox-active metal ion, such as VOPO4. In general one would extend this to a system that is already thermal stable and environmentally benign that can accommodate more two or more lithium (or sodium) per redox-active metal, e.g. V2O5-nH2O aerogels (or delta-phase V4O10-nH2O).
Our research has focused on addressing what is happening at the microscopic level when Li ions are intercalated into aerogels cathodes. We employed V2O5-nH2O as a model system to understand why aerogel-based cathodes fail to retain the high capacities initially afforded by their sparse nature. In particular we addressed the following research questions (summarized by Fig. 1):
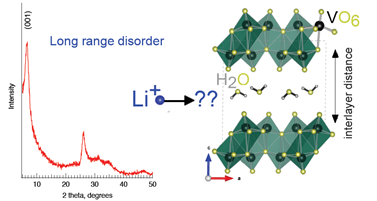
1) how are the Li+ inserted into the aerogel structure?
2) how do the Li+ interact with the crystalline water species required to retain the aerogel form?
Fig. 1: The loss of long-range order makes it difficult to determine how Li+ are intercalated in V2O5-nH2O aerogels. We employed a combination of XPS and PDF to determine the local chemical and geometric structure of V2O5-nH2O aerogel electrodes at various stages of charge and discharge.
Experimental:
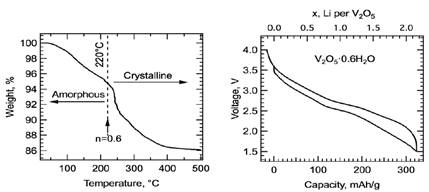
Electrodes: We carefully prepared V2O5-nH2O aerogel electrodes at Binghamton University with the minimal amount of water necessary to retain their amorphous nature (Fig. 2).
Fig. 2: The V2O5-nH2O was heated to 220°C to minimize water content without collapsing the aerogel structure. The resultant electrodes displayed an initially high capacity (> 330 mAh/g) within 4- 1.5V vs. Li+/Li0. Disassembled electrodes at various stages of the first cycle discharge/charge cycle were transferred in inert conditions for XPS and PDF measurements.
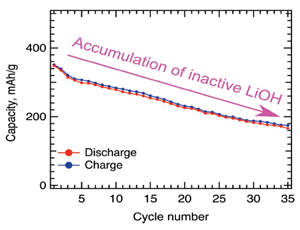
Capacity Retention: The electrodes displayed initially high capacity of over 330 mAh/g corresponding to more than 2 Li+ per V2O5, which halved after only 30 cycles (Fig. 3)
Fig. 3: The initially high capacity of the V2O5-nH2O aerogel electrodes was found to halve over 30 charge/discharge cycles (4.0 – 1.5 V vs. Li+/Li0). XPS and PDF measurements revealed the formation of LiOH species forming upon Li+ insertion, which was only partially decomposed upon Li+ extraction resulting in the accumulation of electrochemically inactive LiOH each cycle.
Local probes: The loss of long-range structure meant that traditional X-ray diffraction (XRD) could not be employed to determine how the structure evolved with Li+ intercalation. Instead we employed a combination of X-ray photoelectron spectroscopy (XPS) at Binghamton University, with Synchrotron-based pair distribution function (PDF) at the new NSLS-II facility at Brookhaven National Laboratory. We prepared a series of electrodes at various stages of discharge/charge during the first cycle (and select cycled samples) to determine the evolution of the local chemical (XPS) and geometric (PDF). We analyzed the PDF data in terms of atomistic models from density functional theory performed at Trinity College Dublin.
Results:
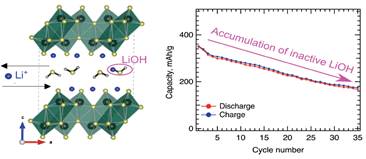
Using our methodology we confirmed that in addition to the expected vanadium redox due to Li+ intercalation into the V2O5 double layers, the inserted Li+ ions also reacts with the tightly bound water to form electrochemically inactive LiOH that accumulates each cycle thereby reducing the capacity.
Implications:
The same water responsible for facilitating the large interlayer distances largely considered beneficial for Li+ transport is also responsible for hindering the capacity retention of aerogel-based multi-electron cathodes for lithium ion batteries.
Summary: These results were published in ACS Applied Materials & Interfaces
Table of Contents Figure: The crystalline H2O molecules required to retain the aerogel structure react with the inserted Li+ ions to form inactive LiOH species that accumulate each cycle thereby explaining the loss of capacity.
Additional studies:
We have also examined Li+ insertion and polaron migration in crystalline V2O5 nanowires. This work built on our previous years this PRF grant studying the evolution of the electronic structure of V2O5 with Li+ (and Ag+, Pb2+) intercalation. The results were published in Nature Communications