Reports: UR455137-UR4: The Strength of Intramolecular Hydrogen Bonds in Fluoroorganic Molecules
Robert E. Rosenberg, Ph. D, Transylvania University
NMR Titration.
The most progress made this summer was on showing how NMR spectroscopy could be used to measure binding energies. After initial forays by three students over previous summers, the fourth attempt proved successful at giving reliable data. As our whole proposal was built around success here, this was an important step forward.
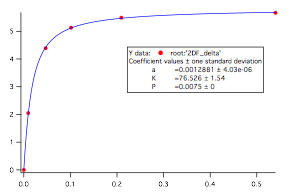
Starting from 1.00 M stock solutions in CCl4, the student was able to make 10 mM solutions of a substituted phenol and add from 10-500 mM of base. The student then recorded the 1H and (when relevant) 19F NMR spectra. We then fit the data to a binding curve using non-linear least squares fitting via IGOR-PRO. Specifically, we plotted the change in chemical shift vs. the concentration of added base. Interestingly, the derived equilibrium constant K was relatively insensitive to the input concentration of phenol. We then converted K to ΔE to get binding energies. A representative data point is shown below in Figure 1.
Figure 1. Graph of change in 19F chemical shift vs. concentration (mM) of added base for addition of DMSO to 2-fluorophenol. The K derived from this fit is the equilibrium constant.
![]() Table
1. ΔE of internal hydrogen
bond of 2-X-phenol as measured by DMSO titration and 1H NMR. Values in kcal/mol.
Table
1. ΔE of internal hydrogen
bond of 2-X-phenol as measured by DMSO titration and 1H NMR. Values in kcal/mol.
F | Cl | Br | OCH3 |
0.73 | 1.47 | 1.54 | 2.71 |
The test case for the method compared the ΔEs for 2-fluorophenol with that for 4-fluorophenol. It was found that ΔΔE (difference in ΔEs) determined by 1H NMR and 19F NMR for both acetone and DMSO as bases were within 5% for the three of the four cases. The fourth case (acetone, 1H NMR) needs to be repeated Furthermore, the value of ΔΔE, –0.75 kcal/mol, is very much in accord with a weak hydrogen bond.
The student performed other studies on 2-chlorophenol, 2-bromophenol, 2-methoxyphenol and their 2-X-6-fluoro- counterparts. As seen in Table 1, above, the 2-chloro- and 2-bromophenols showed slightly stronger binding than 2-fluorophenol and significantly weaker binding than 2-methoxy phenol. These data are consistent with other estimates of these bond energies and helps validate the use of our method.
Synthesis of 2-(fluoromethyl)phenyl]methanol
Scheme 1
Our initial synthesis of 2-(fluoromethyl)phenyl]methanol envisioned several steps starting from some sort of phthallic acid derivative. Indeed, two previous students made some strides here. However, a student this summer found a much better route.
Starting with 2-(hydroxymethyl)phenyl]methanol, the student could selectivtly convert one of the CH2OH groups to CH2Br using HBr. The resulting crystalline solid was purified by column chromatography and treated with TBAF·3.5 H2O to give the desired compound in two steps. The scheme is illustrated in scheme 1, above.
The target compound was then subjected to the NMR titration method above. Very preliminary analysis shows the presence of a very weak to nonexistent hydrogen bond.
Synthesis of 2-(fluoromethyl)phenol
Scheme 2.
Scheme 3
This compound has frustrated all our efforts. Now, having exhausted the work of a fourth student, the 'simple' synthesis has resisted all of our efforts. The basic idea is shown in scheme 2. Not surprisingly, direct reaction of the starting material was complicated by the phenolic OH. So, the strategy was to first protect the phenolic OH. Earlier students were able to selectively add a Boc group and this was accomplished. Then, the plan was to convert the –CH2OH to –CH2F. This was not accomplished. The final step was to remove the Boc group. This was accomplished easily in control experiments as seen in scheme 3.
The latest student successfully showed that model syntheses starting from benzyl alcohol gave benzyl fluoride. Then, the student either used Deoxo-Fluor on the alcohol or converted benzyl bromide to the fluoride using TBAF·3.5 H2O. However, we could not get the Boc-protected compound to fluorinate. Indeed, my latest student could not get it to chlorinate either. The latest attempt was to change protecting groups. Attempts to add an acetate protecting group resulted in acylation of both OH groups..
Training of undergraduates
This is the first year of the grant and I used it to encompass two summers. So, a total of six students have been hired over the last two summers. All students have been either chemistry or biochemistry majors. One of these students graduated and is in pharmacy school. Another graduate is working as a laboratory technician and plans to reapply to dental school. All of the other students are seniors, except one junior. Of these, two plan on going to medical school. The plans of the other two are unclear.