Reports: ND554179-ND5: Fundamental Properties of Two-Dimensional Zeolites
Uwe Burghaus, North Dakota State University
In the meanwhile several inorganic two-dimensional (2D) crystals (analog to the prototypical organic graphene) are known. A technological important example is 2D silica films, called silicatene. Interestingly, doping silicatene with aluminum results in novel 2D zeolite-like materials.
These systems are particularly interesting since until recently only three-dimensional zeolite powders and granules could be studied. Therefore, not much was known about surface properties due to the lack of a 2D analog to zeolites. Recently a surface science procedure to fabricate 2D zeolite-like thin films was developed by Freund’s and Goodman’s groups which opened up a new research field. Now surface properties also for zeolites can be studies in detail using surface science techniques.
The molecular structure of these films is well described in the literature [Freund, Goodman]. However, much less is known about their surface chemistry and catalytic properties. Therefore, the general object of this two-year project was to further characterize the surface chemistry of these systems. In a practical sense and given the nature of this seed grant program perhaps even more important was the second goal, namely to develop skills in our group for fabricating silicatene in the first place.
Specifically we proposed as our objective to synthesize 2D zeolite-like films, deposit Mo clusters on the porous zeolite material, and use thiophene as a probe molecule to map the catalytic activity of the system for hydrodesulphurization (HDS) catalysis. Note that these thin films are grown on Mo single crystal supports. Mo is a well-known catalyst for HDS. The original proposal pointed out that our project will focus on the synthesis of these films which is a significant challenge.
Obviously, a necessary prerequisite of our project is to first synthesize 2D silica films and then aluminum doping these films to fabricate 2D zeolites. Developing that skill will open up for our group a large variety of possible future projects on a new class of materials (inorganic graphene, silicatene) which can potentially attract funding from other sources. (Related NSF proposal will be submitted this year since this non-renewable seed grant is now expired.) Surface science joins more and more with materials science and nano-science. Therefore, “synthesis” skills become important for the next generation of surface chemists which we educate in our laboratories. The participating students will gain those skills. HDS catalysis is a petroleum related research topic.
Unfortunately and in contrast to epitaxial graphene, the nanofabrication of silicatene in ultra-high vacuum (UHV) is rather tricky and cumbersome; a number of variations of preparation receipts can be found in the literature. However, not all parameters were really available from the literature; not all tricks were revealed or known. Our preparation variation, similar to literature, did consist of a thorough UHV cleaning of the Mo(112) support and subsequent formation of an oxygen overlayer structure by oxygen exposure. Next, Si vapor deposition and high temperature oxygen annealing cycles were applied, until the typical hexagonal LEED pattern of silicatene appeared. One of the tricks which allows to fabricate crystalline 2D silica films is using the right starting structure, namely Mo(112)–O rather than clean Mo-surfaces. (A number of different oxygen overlayer structures exist on Mo(112).) The latter results usually in amorphous Si films, only. Similarly, too great annealing temperatures induce defects in silicatene. Too low temperatures appear to inhibit a perfect crystallization of the silica film. And, too much Si (overdosing) results again in an amorphous layer rather than in a crystalline film. In addition, some parameters are difficult to measure precisely such as the Si flux. Similarly, high temperatures are usually difficult to measure accurately. Therefore, the preparation requires some fine-tuning for every experimental setup used. The experimental parameters are given in our first paper as accurately as possible.
In the meanwhile we have succeeded synthesizing silicatene. Despite the complex synthesis, the success of the nanofabrication is, in our opinion however, consistent to the observation of a hexagonal LEED (low energy electron diffraction) pattern. Amorphous films do not generate a LEED pattern; the Mo substrate has a rectangular unit cell opposed to the hexagonal pattern of silicatene. Whereas STM (scanning tunneling microscopy) micrographs provide more details, LEED, AES, and XPS sample the macroscopic surface structure important for catalysis. However, the synthesis is further complicated by reports that oxygen-rich and oxygen-poor structures can be made which apparently cannot be distinguished using LEED. Similarly, atomic defects and Si clusters on top of these films are not easy to identify. Therefore, as a further characterization we collected thermal desorption spectroscopy data of water since the hydrophobicity of the film depend e.g. on the defect density. Indeed, it turned out the hydrophobicity depends on the very details of the film preparation. Only well-ordered films are perfectly hydrophobic. Thus, water TDS can be used as a very sensitive chemical probe technique for film quality when STM characterization is not available. Water TDS is more sensitive than standard LEED. In addition to LEED and TDS, the silica films were characterized by XPS (X-ray photoelectron spectroscopy) and Auger electron spectroscopy, in agreement with prior studies. We succeeded to fabricate silicatene which was the main goal of this project.
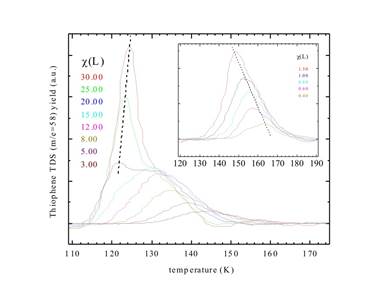
Because samples have now been made successfully we started studying thiophene adsorption and related HDS surface processes. The first publication supported by this grant was already accepted for publication following a peer review process.
Fig. 1: (color online) water TDS and LEED images of silicatene. Although the quality of the LEED images may be comparable, according to TDS, one silicatene surface is hydrophobic (0-th order desorption, low temperature edges lining up) the other one hydrophilic.
Fig. 2: (color online) TDS (thermal desorption spectroscopy) of thiophene adsorption kinetics on silicatene.