Reports: ND554804-ND5: Polyhedral Oil Droplets: Nanoscale Elasticity in Emulsions
Eli Sloutskin, Bar-Ilan University
Moshe Deutsch, Bar-Ilan University
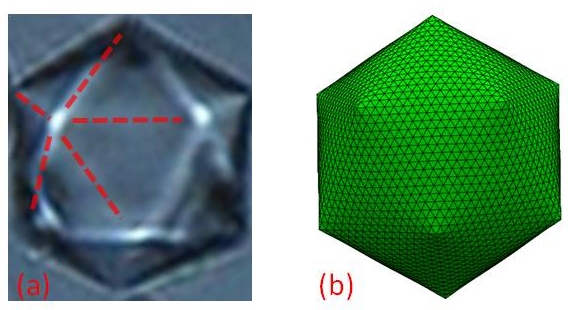
In the absence of external forces, liquid droplets are perfectly spherical, as dictated by the requirement of a minimal surface energy, the product of the droplet's surface area and its surface tension g. Recently, we have discovered an unexpected shape transition: at a certain temperature, T=Td, oil droplets suspended in dilute aqueous surfactant solutions spontaneously adopt a faceted icosahedral shape, while still remaining liquid (Fig. 1a). The physics of this unique transition was the subject of our ACS-PRF-ND funded studies.
Figure 1. (a) A liquid droplet of oil in water adopts an icosahedral shape at TSE<T<Td (bright-field microscopy image). Note the 5 ridges emanating from each vertex (dashed lines). The volume of the droplet is ~5pL. (b) The transition of droplets to an icosahedral shape is fully reproduced by our computer simulations.
Our studies focused on droplets of normal-alkanes, CH3 (CHn-2)CH3 [Cn, 14<n<18], fully-saturated linear hydrocarbons, which are one of the most basic building blocks of organic molecules like oils, alcohols, fatty acids, etc. The droplets were suspended in sub-mM solutions of C18TAB [CH3 (CH17)(CH3)3Br], a common ionic surfactant, bearing an alkyl tail of the same geometry as that of a simple alkane.
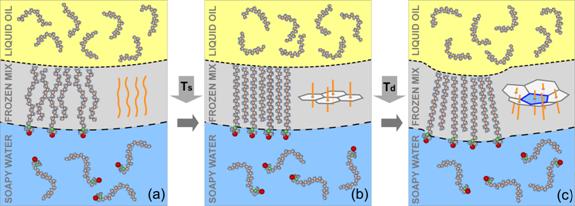
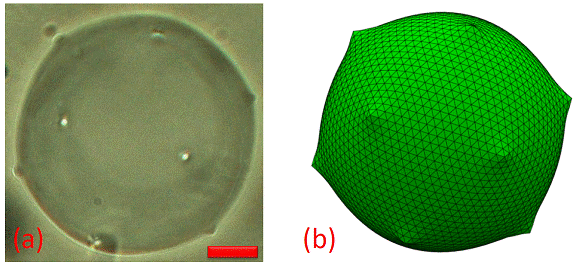
We have established that the faceting transition is driven by the formation at T=Ts>Td of a 2nm-thick crystalline monolayer at the surface of these droplets. This interface-frozen monolayer consists of cocrystallized fully-extended alkane and surfactant molecules, the long axes of which are oriented normal to the oil-water interface (Fig 2). The molecules in this crystalline monolayer are hexagonally packed, and cannot therefore tile the spherical surface of the droplets, without including structural defects. At T>Td, the interfacial tension dominates, so that the droplets retain their spherical shape. However, a dramatic decrease of g(T) is dictated by the thermodynamics of the frozen interface on cooling below Ts. Consequently, at T=Td the shape becomes dominated by the elastic energy of the interfacially-frozen monolayer. Under such conditions, the distortion energy of this quasi-two-dimensional crystal is minimized by forming exactly 12 five-fold defects [molecules with 5, rather than 6, nearest neighbors (NNs); the “topological charge” of such a defect is 6-5=1]. Moreover, for small droplets (R<50mm) the defects buckle, with the droplet adopting the shape of a regular icosahedron (Fig. 1a). We fully reproduce the transition to an icosahedral shape by simple computer simulations, involving only two dimensionless parameters: g/Y and k/YR2, where Y is the two-dimensional Young modulus of the interfacially-frozen lattice and k is its bending modulus. Further simulations of the full dynamics of these transitions are underway. Remarkably, the largest droplets studied (50<R<150mm) do not deform into icosahedra; a spherical shape with exactly 12 protrusions is formed instead (Fig. 3a). As for the smaller droplets, the shape transition is fully reproduced by our computer simulations (Fig. 3b).
Figure 2. Molecular structure cartoons of the mixed C16:C18TAB interfacial monolayer. (a) Molten interfacial monolayer at T>Ts. Yellow, blue, green and red denote C, H, N+, and Br-. C18TAB headgroups are partially water-ionized and hydrated (not shown). (b) Only the interface freezes at T=Ts, forming a crystalline, hexagonally-packed, monolayer of extended, interface-normal, molecules (inset). (c) Buckling of a 5-fold defect in an otherwise hexagonally-packed monolayer, residing at the curved interface of an oil droplet in water. Since a closed surface cannot be tiled by hexagons, the frozen monolayer includes 12 five-fold defects, associated with a very high in-plane stretching energy. This energy is partly relaxed by buckling, which reduces the local radius of curvature. The inset shows the extended linear conformation of the alkane molecules (orange lines), their hexagonal in-plane packing (as in the b inset), and the buckled defect.
At T<Td, the surface tension drops further. At even lower temperatures, T<TSE, the free energy of the interfacially-frozen cocrystallized alkanes drops below their bulk free energy. Under these conditions, the droplets tend to increase their surface area, to minimize their surface energy. Thus, spontaneous splitting of droplets is occasionally observed, reminiscent of the classical spontaneous emulsification, occurring en-route to a microemulsion; however, in contrast with the classical spontaneous emulsification, in our system these phenomena are driven by the interfacial freezing, and thus are temperature-tunable and reversible.
At T<TSE, the icosahedra distort into other shapes, most of which are significantly flattened by buoyancy: hexagons, tetrahedra, rectangles, parallelograms, triangles, and tubules. All of these shapes are consistent with the total topological charge of the defects being 12. Thus, the hexagons include 6 four-fold defects, located at their vertices; the topological charge (i.e., the number of missing NNs) of each of these is 6-4=2. The tetrahedra, the rectangles, and the parallelograms include 4 three-fold defects, of charge: 6-3=3. The triangles include 3 two-fold defects, of charge 6-2=4. Finally, the tubules have 2 holes at their edges, each carrying a topological charge of 6. Remarkably, we observe no shapes inconsistent with this simple defect counting, such as octahedra or pentahedra. A more complete understanding of the shapes forming at T<TSE, such as already achieved for the high temperature phase (TSE<T<Td), is currently under development.
Figure 3. The largest droplets remain spherical, with 12 protrusions formed at the locations of the 12 five-fold defects of the interfacially-frozen lattice. The observed bright-field microscopy image is shown in (a), where the scale bar corresponds to 20mm. The shape is fully reproduced by our computer simulations, (b).
In conclusion, our research unveiled the physical mechanisms behind the unique shape transitions of alkane droplets, for several different alkane chain lengths. The generalization of these results to other alkane:surfactant combinations is in progress. These already-significant results will serve as a basis for a full scale, multi-year, research proposal to be submitted to a funding agency. The ACS-PRF-ND grant has been instrumental in training several graduate students and postdoctoral researchers and allowed developing a new technique for in situ measurements of ultralow g in emulsions, in addition to the significant research results achieved. This new tool and the expertise in studies of emulsions will allow the PIs to expand their research in this area based on solid, well-tested, experimental and simulational foundations.