Reports: UNI553906-UNI5: Surface Selective Laser Spectroscopy Investigation of Photochemistry of Aromatic Compounds at the Air-Water and Oil-Water Interface
Mahamud Subir, PhD, Ball State University
Our continuing effort to understand interfacial processes resulted in new and exciting insights into the equilibrium and photochemical behavior of phenolic compounds at the air-aqueous interface. The following is a summary of some of the progresses we have made:
· Equilibrium Properties of p-nitrophenol and p-nitrophenolate at the Air-Aqueous Interface
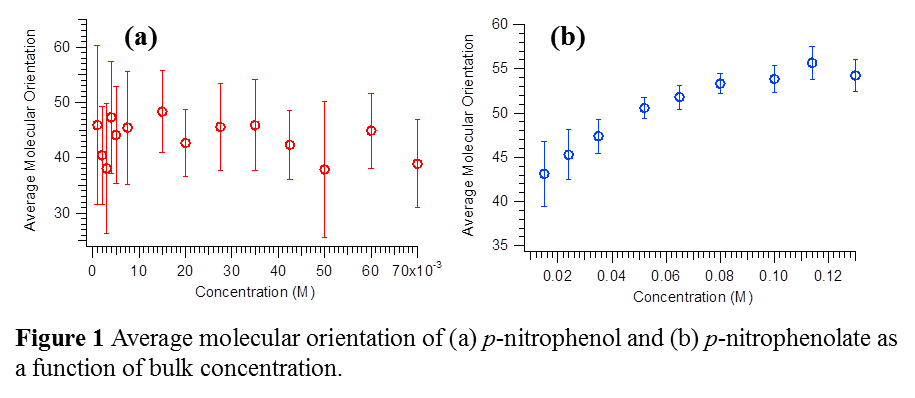
Using SHG we have confirmed that the molecular orientation of p-nitrophenolate (p-NP-), a negatively charged phenolic compound, undergo significant orientation adjustment with an increase in surface population. As surface population is increased, the p-NP- molecules appear to transform from a vertical to a horizontal orientation configuration on average. In contrast, p-nitrophenol (p-NP), which is neutral, does not appear to show significant orientation change within the experimental uncertainty. Figure 1 illustrates the orientational behavior of these two species.
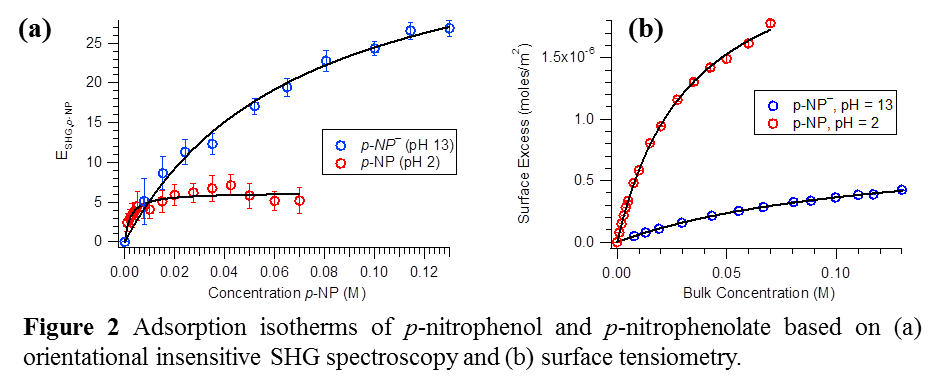
Adsorption isotherms based on orientation insensitive SHG spectroscopy and surface tension measurements have been carried out for both p-NP- and p-NP (Fig. 2). This comparative study provided quantitative understanding of the surface population of p-NP- and p-NP. It is established that compared to pNP–, pNP has a greater affinity for the air-water interface. Moreover, comparing these adsorption affinities to the existing literature values for phenol and aniline, and their conjugate base and acid, respectively, it is found that the -NO2 group plays a major role in driving the phenolic compound to the surface. The source of orientational reorganization in pNP– and the influence of interfacial properties is currently under investigation.
· Determination of Photo-degradation Quantum Efficiency
In order to determine the photo-degradation quantum efficiency, a potassium ferrioxalate based chemical actinometry was implemented. Using this method, we have determined the photolysis quantum efficiencies of p-NP- (pH 13) and p-NP (pH 2). In the bulk solution, the quantum efficiencies of p-NP- and p-NP were determined to be 1.33 (± 0.04) × 10−5 mol×einstein−1 and 3.31 (± 0.08) × 10−5 mol×einstein−1. This indicates that p-NP- is more photo-stable than p-NP in the solution phase.
We have also carried out surface photo-degradation of p-NP- using SHG. While our goal still remains to obtain results with better signal to noise ratio, the kinetic data collected suggests that the photo-degradation rate is slower at the surface. A probable explanation relies on the mechanism of the photo-degradation reaction under study and how the phenolic compound is oriented at the surface. Once confirmed, this result will provide impactful insight into the influence of molecule orientation on interfacial photo-degradation. Currently, practical challenges such as acidification of the surface during the time frame of the photo-degradation are being carefully studied. Also, adjusting the time scale of the reaction by means of radical reaction is being explored.
· Spectral Profile of p-NP- at the air-aqueous interface
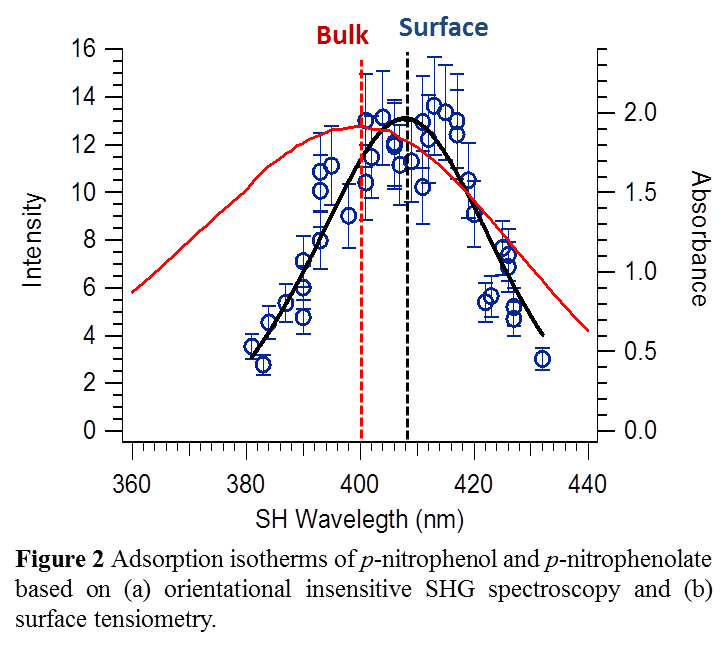
We have measured the surface SHG spectrum of pNP– at the air-aqueous interface (Fig. 3). It appears that pNP– exhibits a slight red shift. This shift is due to the fact that the air-water interface is less polar than neat water. The pNP– SHG spectrum is consistent with its spectral behavior with solvent polarity.
· Impact on Students and the PI
During this project period, one graduate student (Mr. Daniel Headley) was supported by the PRF grant. He has successfully completed his MS degree in Chemistry and is currently pursuing a doctorate degree in Physical Chemistry at Ohio State University. The rigor and the advanced nature of this PRF funded project certainly allowed him to prepare for a higher degree in science. Additionally, we have trained Mr. Ryan Young, a MS candidate, who is currently leading the project. The project has also provided opportunities for three undergraduate students to take part in high impact research. Throughout this year both graduate and undergraduate students in our group participated in ACS and Indiana Academy of Sciences conferences. Two poster presentations and a research talk presented by the students highlighted the unique aspects of this research. With the exposure to innovative research, undergraduate students in the PIs group are motivated to either pursue higher education in Chemistry or find career in industrial settings.
The impact of the funding of this project on the PI is manifold. The preliminary data resulted from this work allowed the PI to recruit new students, initiate collaborations with other faculty members within the department, and extend the project idea to new chemical systems. As a result, the PI is now in position to submit a major federal grant to sustain this exciting interfacial photochemical research. The acquisition of a LCMS unit, which was reported earlier, will certainly facilitate this new research effort. The work also attracted researchers in different scientific communities. Accordingly, the PI has been invited to present research at various academic and national settings. This will certainly allow the PI to share the new scientific findings and bolster his scientific trajectory.