Reports: ND1055315-ND10: Is the Road to Hydrodesulfurization Paved in Bronze?
Sarbajit Banerjee, PhD, Texas A&M University
Overview of Goals and Summary of Research Accomplishments: The primary focus of our ACS PRF grant is to (i) elucidate the distinctive electronic structure of the edges of MoS2 thought to be of critical importance for its catalytic activity; and (ii) develop intercalated MoS2 phases and map their catalytic activity towards hydrodesulfurization. Over the project period, we have made tremendous progress on both goals. Synthetic methodologies have been developed that yield well-defined MoS2 platelets integrated onto carbon fiber substrates as well as mesoporous MoS2 thin films with an ordered geometry. The edge electronic structure has been mapped using Mo S L-edge X-ray microscopy measurements. Extensive density functional theory calculations have allowed for identification of spectral signatures of edge sites and enabled the elucidation of the specific states that contribute to the distinctive edge electronic structure. Several topochemically intercalated phases of MoS2 have been prepared and evaluated for catalytic activity in the hydrodesulfurization of dibenzothiophene as a model compound.
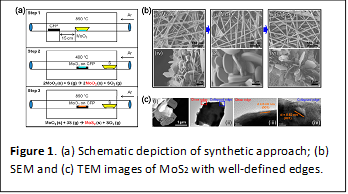
Synthesis of Well-Defined Nanostructured MoS2 Architectures: Two distinctive methodologies have been developed in order to create mesoscale architectures of MoS2. In the first approach, a sequential synthetic method comprising vapor transport, reduction, and topochemical sulfidation has been developed for creating 3D arrays of MoS2 nanosheets directly integrated onto carbon fiber paper (CFP) substrates (Choi et al. ACS Catalysis, 2016, 6, 6246–6254).The sulfidation process results in a high density of edge sites along both the edges and the basal planes of MoS2. Figure 1 depicts the well-defined edges of MoS2 nanostructures obtained by this method. The sulfidation step results in the formation of nanoscale crystallites held together as flakes. The carbon fiber paper promotes both ionic and electronic conductivity for the supported catalysts. In a second approach, a sol—gel route has been used to template the growth of MoO3 thin films infiltrated between a self-assembled layer of polystyrene beads. Carbonization of the polystyrene bead template followed by hydrothermal sulfidation yields open-framework mesoporous arrays of MoS2 as depicted in Figure 2. One fundamental question we have been examining in some detail is how the catalytic activity of amorphous MoS2 evolves as a function of temperature (and is the focus of an article that will be submitted for publication in the next few weeks). With increasing temperature MoSx clusters are cross-linked to form nanocrystalline MoS2 domains. The development of synthetic methods yielding MoS2 nanostructures with well-defined edge sites has been critical to both aims of this project and has enabled detailed studies of edge electronic structure as well as subsequent intercalation with cations as a means of modifying catalytic activity.
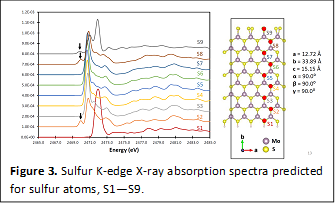
Elucidation of Edge Electronic Structure: The coordinative unsaturation of the MoS2 edge sites, particularly the stabilization of dimers at sulfide rich (10-10) surfaces, is thought to be of critical importance in mediating catalysis. Density functional theory calculations have been performed for large supercells of single-layered MoS2 and have enabled identification of the specific electronic states at the edges of MoS2 that are believed to underpin the catalytic activity of this material. Sulfur K-edge X-ray absorption spectra have been modeled as indicated in Figure 3. Very distinctive spectral features are predicted for the edge S atoms S1 and S9. A sharp red-shifted resonance at 2472 eV is predicted in S K-edge X-ray absorption spectra for edge electronic states.
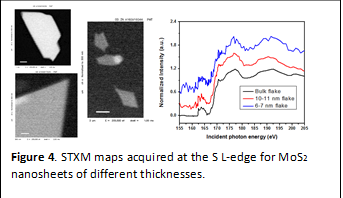
Interestingly, the next-to-nearest neighbors are characterized by distinctive pre-edge transitions. Scanning transmission X-ray microscopy measurements have yielded high-spatial-resolution S L-edge maps indicating the distinctive electronic structure at ripples and edges of MoS2 (Fig. 4). Ultra-thin flakes that span only a few layers in thickness are characterized by distinct pre-edge features in the 155—162 eV region. S K-edge STXM imaging is currently being pursued at beamline 10ID of the Canadian Light Source. The calculated spectra and the ability to obtain high spatial resolution at the S K-edgesuggest that it should be possible to clearly understand how edge reactivity evolves during a catalytic reaction or in “modified” or interfaced catalysts that show superior reactivity as compared to bare MoS2.
Preparation of Intercalated MoS2: The nanostructured MoS2 architectures depicted in Figures 1 and 2 have been intercalated with various cations using a number of different topochemical methods. Reaction with n-butyllithium has been used to prepare LixMoS2, whereas reaction with n-butylmagnesium has been used to intercalate Mg cations. The expanded LixMoS2 phase can be further exchanged for other cations by stirring with metal salts. Ion-exchanged CoxMoS2 and NixMoS2 have been stabilized by this route. The intercalated materials have been thus far characterized by powder X-ray diffraction, which indicates a clear modulation of the interlayer spacing.
Screening of Catalytic Activity: A high-pressure hydrothermal vessel has been procured and commissioned for evaluation of hydrodesulfurization reactions. The reactions are currently being performed at 300°C under a ca. 7 MPa H2 ambient. A gas chromatography procedure has been developed to monitor the reactant concentration as a function of time. The NixMoS2 catalysts show the best activity thus far of the intercalated molybdenum sulfide bronzes.
Ongoing Efforts: Our current efforts are focused on elucidating the electronic structure origin of the distinctive X-ray absorption fine structure features observed for few-layered MoS2 and to examine how these features evolve with topochemical cation intercalation or during catalysis. Furthermore, our efforts are focused on greatly expanding the library of available MxMoS2 phases with intercalated cations and to map their catalytic activity for the hydrodesulfurization of dibenzothiophene. The intercalated phases, particularly those that show promising catalytic performance, will be extensively characterized using local structure probes such as X-ray absorption fine structure spectroscopy and element-specific X-ray absorption near-edge structure spectroscopy. Compositions showing particular promise will be prepared in large amounts to facilitate neutron diffraction experiments.