Reports: UR353234-UR3: Novel Topologically Constrained Transition Metal Complex Oxidation Catalysts
Timothy J. Hubin, Southwestern Oklahoma State University
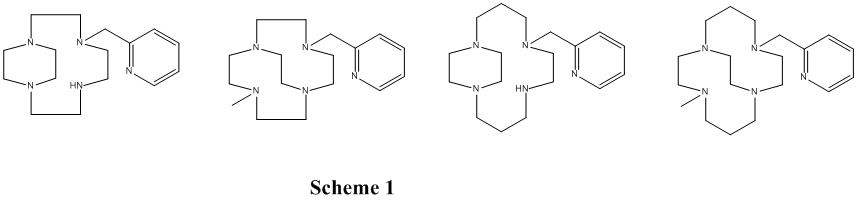
During the grant period for this report we concentrated our efforts on fully characterizing and exploring the oxidation catalyst potential of several pyridine pendant armed complexes previously prepared (Scheme 1).
Transition metal complexes, including Fe, Mn, and Cu of these ligands were synthesized successfully, either from the metal chloride or acetate salts. Complete chemical characterization of these complexes were carried out.
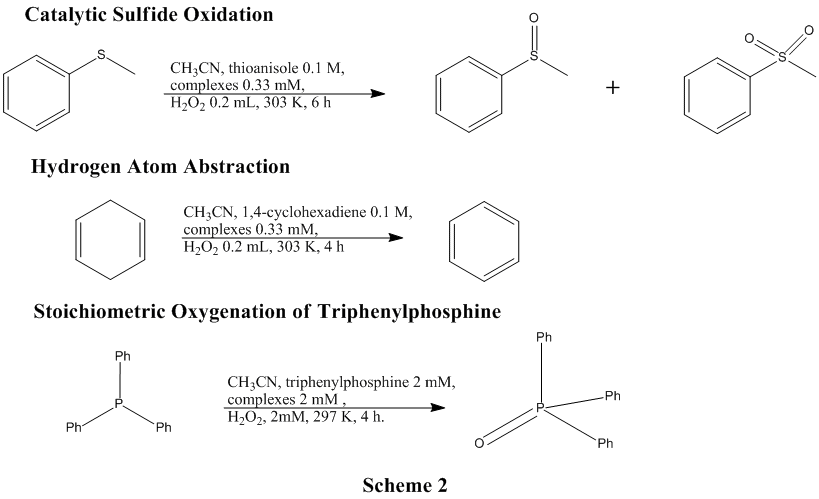
Various catalytic oxidation reactions have been run for each of these compounds with the help of collaborator Guochuan Yin (Scheme 2).
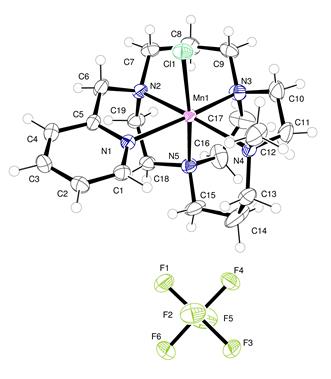
Conclusions were that the cross-bridged cyclam ligand manganese complex was the best catalyst, with excellent selectivity and mild oxidation potentials. (Figure 1, Table 1).
Figure 1. Asymmetric unit of [Mn(PyMeEBC)Cl]PF6 with atoms drawn as 50% probability ellipsoids. Atom colors: Mn pink; Cl green; C gray; N blue; F yellow-green.