Reports: ND554543-ND5: Metal-Organic Frameworks as Gas-Phase Heterogeneous Catalysts: Understanding Active Sites for Oxidation and Hydrogenation Reactions
Donna A. Chen, University of South Carolina
Natalia B. Shustova, PhD, University of South Carolina
Overview
We are investigating metal-organic frameworks (MOFs) for the development of new catalytic and adsorbent materials, given that MOFs provide the ability to precisely control the architecture of active metal sites. The reactive metal centers in MOFs consist of unsaturated metal sites (UMS), and the exploration of chemical activity at these UMS is critical for understanding catalysis and adsorption on MOFs. During the second year of the grant, we have synthesized bimetallic and monometallic MOFs of interest (PI: Shustova) and studied their oxidation states and electronic properties in the presence of adsorbates by X-ray photoelectron spectroscopy (PI: Chen). Our key findings from this reporting period are as follows: adsorption and desorption of molecules like O2 and H2O at Cu+1 UMS sites caused reversible changes in the electronic structure near the Fermi level for Cu-containing MOFs; and Co-Cu bimetallic MOFs exhibited densities of states near the Fermi level that are similar to semiconductors, whereas pure Cu, pure Co, Zn-Cu and Ni-Cu MOFs showed densities of states characteristic of insulators. One manuscript on Cu-containing MOFs has been published in the Journal of Physical Chemistry C, and another manuscript on the bimetallic MOFs is in preparation and will be submitted by December 2016.
Current Progress
Design, Synthesis, and Characterization of MOFs
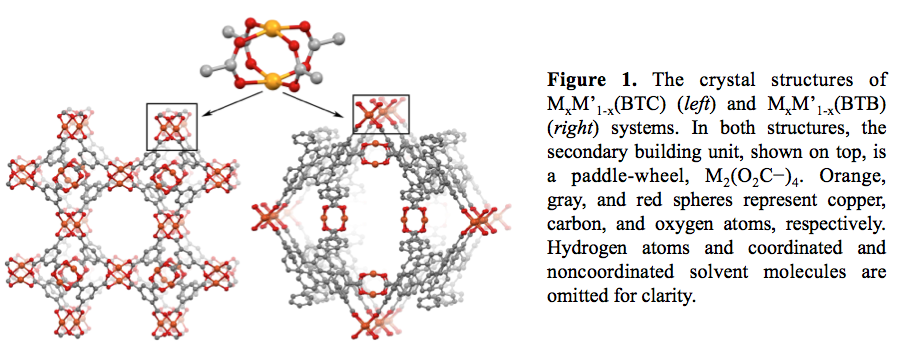
The choice of the metal-organic system was dictated by following criteria: (i) high thermal stability, (ii) ability to retain crystallinity after activation, (iii) ability to preserve crystallinity after exposure to air during transfer to the X-ray photon electron spectroscopy (XPS) chamber, and (iv) preservation of integrity during immobilization of the second metal for preparation of bimetallic systems. As a result, two types of metal-organic frameworks (MOFs) were chosen:
1. MxM'1-x(BTC) system (M = Cu; M' = Cu, Zn, and Co; H3BTC = benzene-1,3,5-tricarboxylic acid, Figure 1).
2. MxM'1-x(BTB) system (M = Cu, Ni; M' = Cu, Ni, and Co; H3BTB = 1,3,5-tris(4-carboxyphenyl)benzene, Figure 1).
The first step in preparation of the chosen MOF systems was ligand synthesis. The linker H3BTC is commercially available while the linker, H3BTB, was prepared using a two-step procedure (Scheme 1). The choice of transmetallation conditions for preparation of bimetallic MOFs and activation conditions were developed separately for each system. In general, preparation of monometallic MOFs was performed by the solvothermal synthesis. The bimetallic MxM'1-xMOFs were prepared using two different synthetic routes: (Route 1) direct synthesis, i.e., two metal salts, MX and M'Y, were introduced into the reaction mixture simultaneously and (Route 2) solid state metathesis of the prepared monometallic M-MOF in a saturated solution of the M'Y salt. Within one type of a MOF system, monometallic M-MOFs and bimetallic MxM'1-x-MOFs were isostructural.
After MOF preparation, the activation conditions were tuned to preserve MOF integrity after heating. In general, to remove organic solvent from porous MOFs two procedures were used: (1) heating of as-synthesized samples under vacuum using the Schlenk line and (2) exchange of an as-synthesized MOF sample with more a volatile solvent (e.g., dichloromethane) followed by heating under vacuum. The prepared MOF samples underwent comprehensive characterization by single crystal and powder X-ray diffraction, thermogravimetric analysis, Fourier transform infrared spectroscopy, inductively coupled plasma mass-spectrometry, energy-dispersive X-ray spectroscopy, and gas sorption analysis.
Investigation of Electronic Properties by XPS
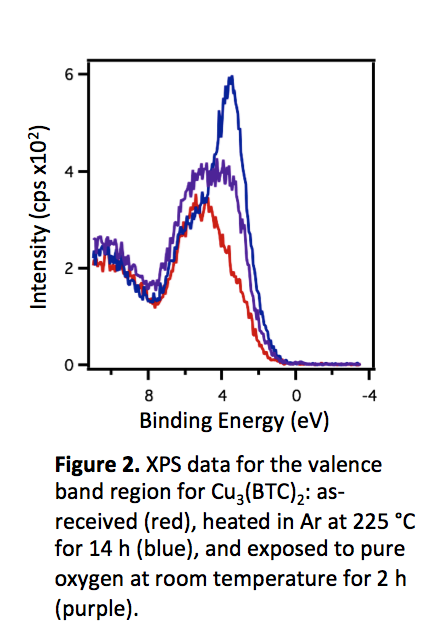
XPS studies of Cu3(BTC)2 demonstrated that there is a significant change in the density of states near the Fermi level when this MOF is activated by heating in argon at 498 K to remove the water and residual solvent molecules coordinated to the UMS. During this activation procedure, the majority of the Cu+2 species is reduced to Cu+1, and a marked change in the valence band spectrum is shown in Figure 2. Furthermore, exposure of the MOF to O2 as an adsorbate caused the density of states to diminish again, as Cu+1 was reoxidized to Cu+2. Thus, reversible changes in the electronic structure were observed from the binding and desorption of adsorbates.
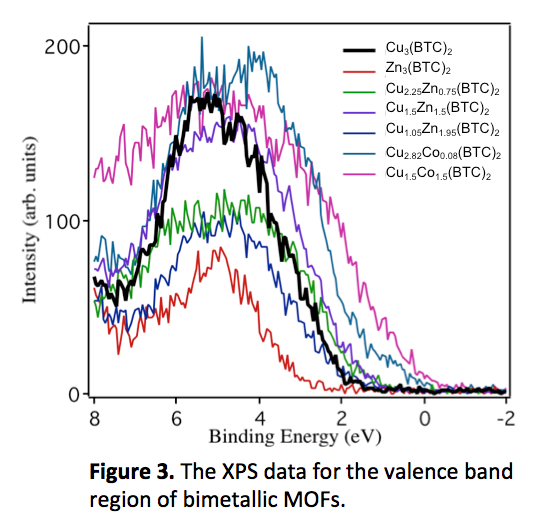
A high density of states near the Fermi level was also observed for the bimetallic Cu1.5Co1.5(BTC)2, in which the oxidation state of Cu was primarily Cu+2. Figure 3 illustrates that the intensity of the valence band spectrum is nonzero at the Fermi level (binding energy=0) for the Cu1.5Co1.5(BTC)2 MOF. In fact, the shape and position of the valence band spectrum is characteristic of a semiconductor; in contrast, the pure Cu3(BTC)2 exhibits a lack of intensity at the Fermi level that is characteristic of an insulator. This change in density of states was observed even for the Cu2.82Co0.08(BTC)2 which has Co concentration of only 2-3%. However, incorporation of Zn+2 into the pure Cu-containing MOF at any concentration did not shift the valence band edge appreciably from the position for Cu3(BTC)2 (Figure 2). In addition, the pure Cu3(BTB)2 and bimetallic CuxCo3-x(BTB)2 did not show increased density of states near the Fermi edge, indicating that the change in electronic properties with Co+2 substitution into Cu3(BTC)2 is unique to this specific metal and ligand combination.
Ongoing Research
Density functional theory studies with a collaborator (Andreas Heyden) are currently underway to help understand how the electronic properties are altered in the CuxCo3-x(BTC)2 system by the introduction of Co+2. Future efforts in preparation of bimetallic MOFs will be focused on synthesis of the stable Zr-based systems, in which catalytically active cites will be created by extension of hexameric Zr metal nodes with metals such as Co, Ni, or Cu. XPS experiments will address the binding of adsorbates and associated changes in electronic properties for the bimetallic MOFs. Crystalline monometallic and bimetallic MOF films have also been prepared for infrared spectroscopy and XPS studies of adsorbate binding.
Impact of Grant on PIs' Research Program
This work has already had a major impact on future research directions in the Chen and Shustova groups; a proposal has been submitted to the National Science Foundation on tailoring the electronic properties of MOFs through the choice of ligand, metal centers and structure. Four female graduate students and one female undergraduate student have participated in this project and had the opportunity to be involved in these new research directions.