Reports: DNI354834-DNI3: Upgrading Polyethylene: Copolymerization of Ethylene and Polar Vinyl Monomers Facilitated by Lewis Acid Appended Ni and Pd Catalysts
Loi H. Do, PhD, University of Houston
2016 PRF Annual Report
Project Background: Polyolefins are the most common synthetic polymers used today, with an annual output of approximately 70 billion kilograms. Functional copolymers, such as those comprising ethylene and polar olefins such as acrylate, acrylamide, or vinyl acetate, are particularly useful materials because they possess better processibility and compatibility compared to non-polar polyolefins. Currently, the only practical synthetic methods to obtain functional polyolefins are through free radical processes, which are efficient for large-scale manufacturing but do not provide good control over the polymer microstructure. Recently, there have been some successes in using late transition metal complexes to catalyze the copolymerization of ethylene and polar olefins. Unfortunately, such systems suffer from at least one or more of the following drawbacks: low catalyst activity, low polymer molecular weight, and/or low polar monomer incorporation. The Do research group has initiated a program to investigate the application of outer sphere influences to facilitate the coordination-insertion polymerization of polar olefins. In this report, we describe the work that we have performed during the ACS-PRF funding period from 09/01/2015 to 08/31/2016.
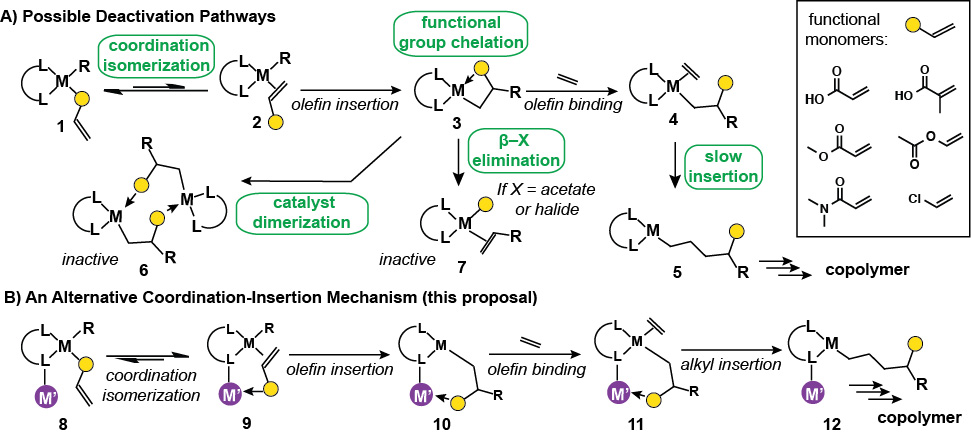
Nickel Complexes With Pendant Lewis Acids. The difficulty in achieving efficient copolymerization of olefins by metal catalysts is the tendency of polar olefins to form chelated structures upon insertion into metal–alkyl bonds. These metallocyclic intermediates are kinetically stable and typically require high pressures of ethylene to ring-open. To avoid this inherent reactivity, our lab has designed new catalyst constructs that will allow the placement of Lewis acidic cations in close proximity to the active metal center. This secondary metal ion will aid in both the binding and insertion of polar olefins by engaging in Lewis acid-base interactions with the monomer so that the polar group does not interfere with polymerization (Scheme 1).
Scheme 1.
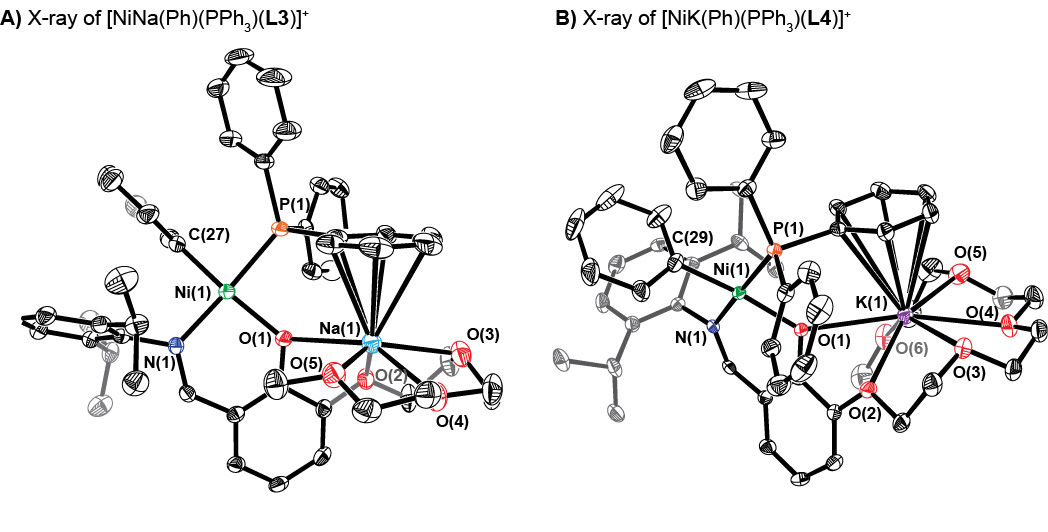
Our first-generation catalysts were based on the phenoxyimine ligand framework. To provide a binding site for alkali metal ions, we introduced a polyethylene glycol (PEG) chain to the ortho position of the phenol ring, giving rise to a series of ligands HLX (where X = 0, 2–4 based on the length of the PEG chain). These ligands can be readily prepared starting from 2,3-dihydroxybenzaldehyde, which can be alkylated with an appropriate PEG unit and then condensed with a bulky aniline to formed the desired compound. Metallation of HLX by reaction with [Ni(Br)(Ph)(PPh3)2] affords [Ni(Ph)(PPh3)(LX)]+ (NiLX) in good yields. Solution titration studies show that the addition of M+ (Li+, Na+, or K+) salts to NiLX leads to formation of both heterobimetallic [NiM(Ph)(PPh3)(LX)]+ (NiMLX) and heterotrimetallic [Ni(Ph)(PPh3)(LX)]2M+ (Ni2MLX2) species, the ratio of the two species in solution are most likely dictated by the size of the alkali cation and the length of the PEG chain. X-ray structural characterizations were obtained for [NiNa(Ph)(PPh3)(L3)]+ and [NiK(Ph)(PPh3)(L4)]+ (Figure 1), which confirmed that the nickel and alkali metal ions are bridged by the phenolate group of the ligand.
Figure 1.
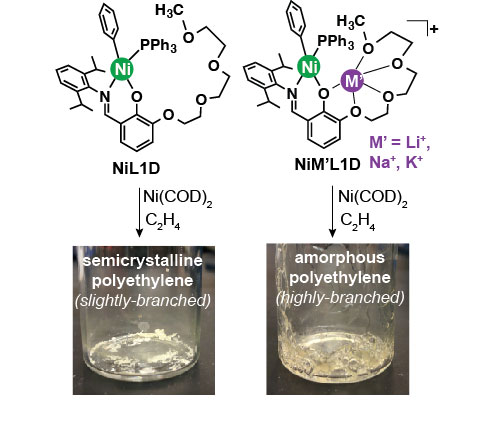
The nickel complexes prepared were then tested in ethylene polymerization. All of the complexes were dissolved in toluene, activated by treatment with [Ni(COD)2] (COD = 1,5-cyclooctadiene) to scavenge triphenylphosphine, and then exposed to 100 psi of ethylene in a glass reactor (Figure 2). Both the monometallic and bimetallic complexes yielded polyethylene, but distinct differences were observed between the two. The addition of Li+, Na+, or K+ to NiLX can result in up to twenty-fold increase in catalyst activity as well as enhancement in polymer molecular weight and branching frequency. The catalyst activity of the NiMLX complexes appear to correlate with the binding affinity of M+ to NiLX, which suggests that the active catalyst is most likely the mixed nickel-alkali metal complex. The NiMLX catalysts are single site (Mw/Mn ~2) and non-living (i.e. no increase in polymer molecular weight by increasing polymerization time). This work is significant because it is the first systematic study of the effect of alkali cations on olefin polymerization catalysts and demonstrates that Lewis acids can have a dramatic influence on the polymerization process. Studies are currently undergoing to investigate the competency of the NiMLX complexes to promote the polymerization of ethylene with polar olefins such as methyl acrylate and vinyl acetate. Additionally, we are also exploring novel ligand platforms to obtain site-differentiated catalysts for olefin polymerization that can overcome the deficiencies of existing catalyst systems.
Figure 2.
The results from our studies of the NiMLX complexes raised an intriguing question regarding the extent to which electronic vs. steric factors influence the catalyst behavior. Working in collaboration with Prof. Robert Szilagyi at Montana State University, we are applying X-ray absorption spectroscopy and density functional theoretical calculations to investigate how the presence of the alkali cations in the nickel phenoxyimine complexes alter the electronic and structural features of the parent species and their possible relevance to polymerization. We aim to develop a robust method to characterize such heterometallic systems in order to acquire a better understanding of structure-activity relationships.
Impact of PRF Support. The PRF Doctoral New Investigator Grant has provided critical seed money to support our work in olefin polymerization. Our research has resulted in a publication in the Journal of the American Chemical Society and several others that are in preparation. This grant has provided partial support of several graduate students in the lab working on various aspects of the project. The grant has also allowed the PI to attend the Organometallics Gordon Research Conference to present our findings in this work.